目前聚变堆增殖包层候选结构材料包括低活化铁素体/马氏体(RAFM)钢和氧化物弥散强化(ODS)钢等[3~5]。其中,Cr含量在9%~12% (质量分数)之间且具有bcc结构的RAFM钢,如中国低活化马氏体(CLAM)钢,具有良好的抗辐照肿胀能力、高导热系数及较成熟的工业化生产基础等优势[6,7]。然而由于基体bcc结构的本质特性,RAFM钢的最高服役温度均不能高于550 ℃。因此,在RAFM钢的基础上,通过机械合金化过程中加入Y2O3颗粒等手段制备出新一代高性能材料ODS钢,如低活化9Cr-ODS钢等。该制备工艺在细化晶粒的同时引入了高体积分数的弥散纳米氧化物(如Y-Ti-O),使得ODS钢具有优异的高温蠕变强度、抗辐照性能、室温低周疲劳性能和热稳定性[8~15]。
与裂变反应堆不同,未来聚变反应堆如CFETR等将采用高温高压水作为冷却介质,其典型的设计出口温度为325 ℃、压力为15.5 MPa。然而,对聚变用低活化结构材料在这种设计工况下的腐蚀行为研究,至今为止仅见针对低活化钢F82H钢的少量报道[27],而对低活化ODS钢的研究尚未见报道。因此,本工作以国产化低活化9Cr-ODS钢为材料,研究其在CFETR冷却介质典型出口温度下的腐蚀行为,阐明微观腐蚀机理。同时,选取一种典型的RAFM钢(中国低活化马氏体(CLAM)钢)进行对比,研究其耐腐蚀性能的异同,以期为CFETR的设计和建造提供数据支撑。
1 实验方法
1.1 实验材料
表1 9Cr氧化物弥散强化(9Cr-ODS)和中国低活化马氏体(CLAM)钢的化学成分 (mass fraction / %)
Table 1
| Steel | C | Si | Cr | Mn | W | Ta | Y | O | Ti | V | S | P | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9Cr-ODS | 0.1 | - | 9.31 | - | 2.04 | - | 0.26 | 0.22 | 0.21 | - | - | - | Bal. |
| CLAM | 0.091 | 0.04 | 8.93 | 0.49 | 1.51 | 0.15 | - | - | - | 0.15 | 18 × 10-4 | 30 × 10-4 | Bal. |
Note: ODS—oxide dispersion-strengthened, CLAM—Chinese low activated martensitic
表2 9Cr-ODS和CLAM钢的热处理工艺
Table 2
| Steel | Normalizing temperature / oC | Normalizing time / min | Tempering temperature / oC | Tempering time / min |
|---|---|---|---|---|
| 9Cr-ODS | 1050 | 60 | 800 | 60 |
| CLAM | 980 | 30 | 760 | 90 |
1.2 腐蚀实验
采用线切割从2种钢板材中切取尺寸为15 mm × 10 mm × 2 mm的片状腐蚀试样,然后采用400~2000号SiC砂纸依次打磨样品表面,使用粒度为2.5 μm的金刚石研磨膏进行抛光,并采用去离子水和无水乙醇进行超声清洗、烘干。
腐蚀实验在温度为325 ℃、压力为15.5 MPa的高温高压水腐蚀回路中进行,采用电导率为0.1 μS/cm的超纯水并控制溶解氧(dissolved oxygen,DO)始终低于10 × 10-9。腐蚀回路装置如图1所示。该装置主要由炉体、真空泵和控制箱3部分构成,具有良好的密封性和耐高温腐蚀性能。腐蚀实验时间节点为200、500和1000 h。腐蚀实验分为2种状态进行:一种是在静态高温高压水中进行;另一种是在动态高温高压水中进行,流速为5 mL/min。每个状态下每种钢均采用2个平行样品。
图1
1.3 腐蚀前/后质量变化及微观分析
腐蚀实验前采用游标卡尺精确测量样品尺寸以获得面积数据。为了评估材料的氧化程度,采用精度为0.01 mg的XS105DU电子天平测量质量变化。使用Smartlab型X射线衍射仪(XRD)分析表面氧化物物相组成。同时,采用配备有能谱仪(EDS)的MIRA3-LMH场发射扫描电子显微镜(SEM)分析氧化层厚度、结构及成分;采用ESCALAB Xi+型X射线光电子能谱仪(XPS)分析氧化层外表面元素的价态;采用JXA-iHP200F型电子探针(EPMA)分析氧化物的元素分布。利用Talos 2100 型透射电子显微镜(TEM)进行选区电子衍射(SAED)分析,电压为200 kV。氧化膜界面的TEM试样使用Helios 5CX型聚焦离子束(FIB)制备。使用FactSage Education 8.3软件计算在325 ℃、15.5 MPa条件下各种氧化物的标准Gibbs自由能。
2 实验结果
图2
图2
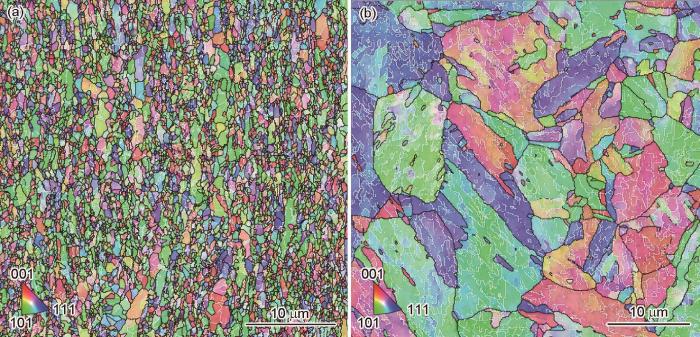
9Cr-ODS钢及CLAM钢基体晶粒尺寸的EBSD像
Fig.2
EBSD maps showing matrix grain size of 9Cr-ODS steel (a) and CLAM steel (b)
2.1 氧化增重
图3
图3
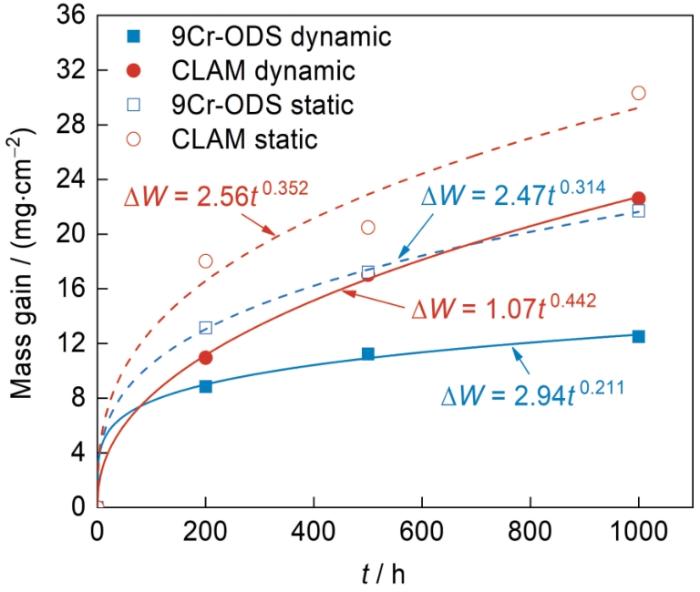
9Cr-ODS和CLAM钢在静态和动态高温高压水中的氧化增重曲线
Fig.3
Oxidation mass gain (ΔW) curves of 9Cr-ODS and CLAM steels in static and dynamic high temperature and high pressure water (t—time)
采用下式对2种钢的增重数据进行拟合[30]:
式中,ΔW为钢的质量变化,mg/cm2;kp为氧化速率常数,mg/(dm2·h n );t为暴露时间,h;n为时间指数,无量纲,用于评估增重与时间的依赖关系,即n越低,增重速率越低。利用
此外,kp越大表示初期腐蚀速率越快。在静态腐蚀条件下,2种钢的kp相差不大;而在动态条件下,9Cr-ODS钢的kp为2.94,显著高于CLAM钢的1.07。
2.2 XRD物相表征
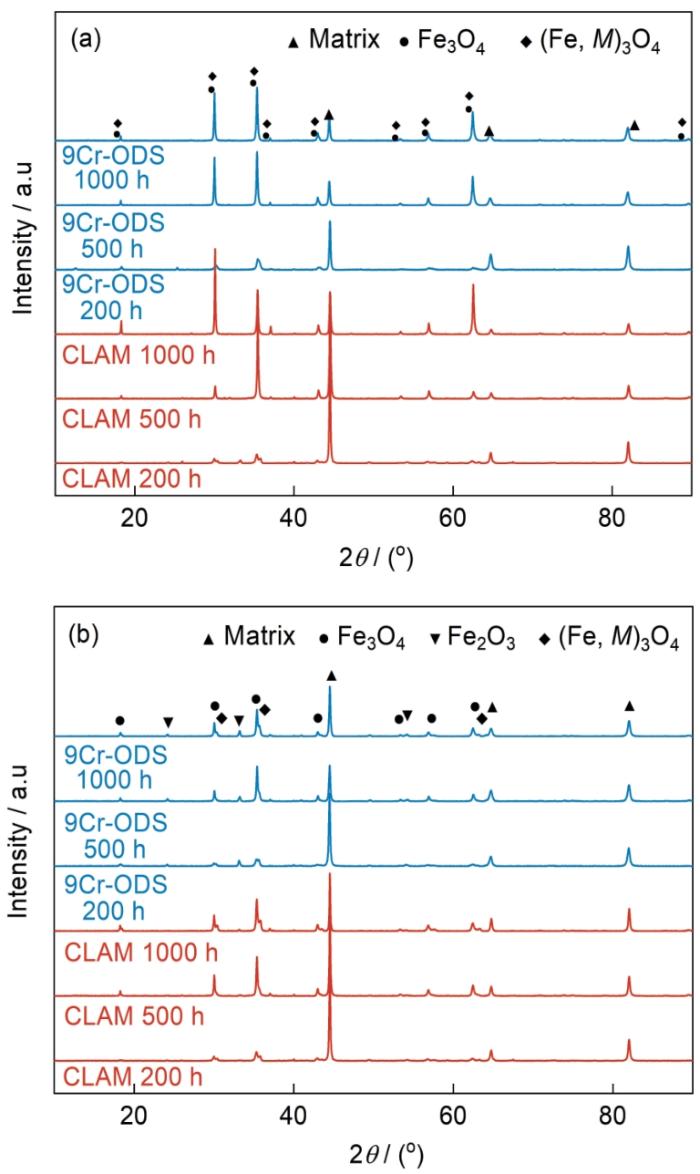
图4
图4
9Cr-ODS钢和CLAM钢在静态和动态高温高压水中腐蚀后的XRD谱
Fig.4
XRD spectra of 9Cr-ODS and CLAM steels after corrosion in static (a) and dynamic (b) high temperature and high pressure water
2.3 表面腐蚀形貌及成分
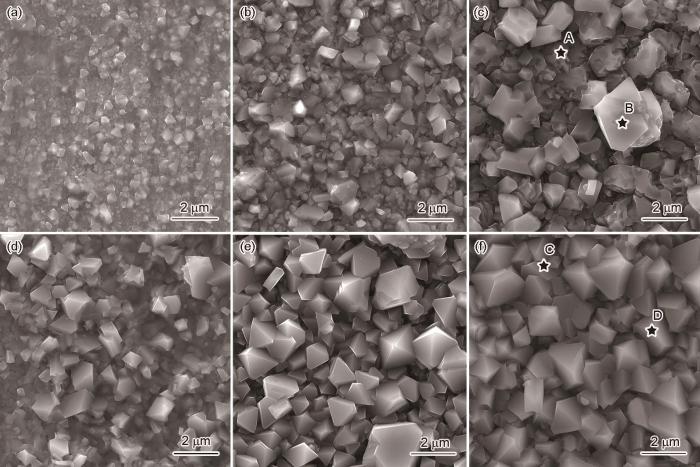
图5为静态条件下腐蚀不同时间后9Cr-ODS和CLAM钢表面腐蚀产物的SEM像。由图可见,随时间的延长,2种钢表面氧化物颗粒的尺寸逐渐增加,但9Cr-ODS钢的表面形貌与CLAM钢存在明显的差异。9Cr-ODS钢腐蚀200 h后,表面氧化物颗粒平均直径在200 nm左右(图5a);腐蚀500 h后,氧化物颗粒变得更为规则,呈现八面体结构,尺寸增大至1 μm左右(图5b);腐蚀至1000 h后,部分颗粒进一步长大至1.5 μm,剩余颗粒的直径和结构基本不变,但尖锐的边角变得更为圆滑(图5c)。CLAM钢在腐蚀200 h后,表面同样覆盖有八面体结构的氧化物颗粒,但尺寸明显更大,在1~2 μm范围(图5d);随腐蚀时间延长至1000 h,CLAM钢的氧化物颗粒尺寸逐渐增大至3~4 μm,但长大趋势有所减慢(图5e和f)。
图5
图5
静态条件下腐蚀不同时间后9Cr-ODS和CLAM钢表面腐蚀产物的SEM像
Fig.5
Surface corrosion product SEM images of 9Cr-ODS (a-c) and CLAM (d-f) steels after corrosion in static condition for 200 h (a, d), 500 h (b, e), and 1000 h (c, f)
表3 图5中标记点的EDS结果 (mass fraction / %)
Table 3
| Steel | Point | Fe | Cr | O |
|---|---|---|---|---|
| 9Cr-ODS | A (Matrix) | 66.00 | 14.56 | 19.45 |
| B (Large-size oxide particle) | 38.28 | 0.90 | 60.49 | |
| CLAM | C (Matrix) | 58.69 | 4.87 | 36.44 |
| D (Large-size oxide particle) | 49.80 | 1.00 | 49.19 |
图6为动态条件下腐蚀不同时间后9Cr-ODS和CLAM钢表面腐蚀产物的SEM像。与静态条件下相比,2种钢在动态条件下的表面腐蚀形貌有明显不同。腐蚀不同时间后,样品表面覆盖的氧化物颗粒数量减少、尺寸增大。其中,9Cr-ODS钢在腐蚀200 h后,氧化物颗粒相对较为稀疏、尺寸约为200 nm (图6a);500 h后,部分氧化物颗粒尺寸长大,形状规则,呈现八面体结构,并可观察到一些类似絮状的产物(图6b);1000 h后,氧化产物尺寸进一步长大(图6c)。CLAM钢在腐蚀200 h后氧化物颗粒数量变少,尤其是较大尺寸的颗粒尺寸占比降低(图6d)。随时间延长(图6e和f),与 9Cr-ODS钢相比,CLAM钢表面氧化物颗粒平均尺寸更大,这与腐蚀增重曲线结果一致。
图6
图6
动态条件下腐蚀不同时间后9Cr-ODS钢和CLAM钢的表面腐蚀产物形貌
Fig.6
Surface corrosion product SEM images of 9Cr-ODS (a-c) and CLAM (d-f) steels after corrosion in dynamic condition for 200 h (a, d), 500 h (b, e), and 1000 h (c, f)
表4 图6中标记点的EDS结果 (mass fraction / %)
Table 4
| Steel | Point | Fe | Cr | O |
|---|---|---|---|---|
| 9Cr-ODS | A (Matrix) | 46.66 | 7.82 | 45.52 |
| B (Large-size oxide particle) | 38.11 | 3.74 | 58.15 | |
| CLAM | C (Matrix) | 46.51 | 9.06 | 44.43 |
| D (Large-size oxide particle) | 31.89 | 7.93 | 61.19 |
2.4 XPS分析结果
采用XPS对水腐蚀后样品表面产物进行元素价态分析,以进一步确认氧化物的类型,如图7所示。Fe2p3/2的XPS结合能分析结果显示,腐蚀至1000 h后,9Cr-ODS钢和CLAM钢在静态条件下的峰位值为710.4和710.3 eV,在动态下为710.6和710.7 eV,均接近于Fe3O4的标准结合能710.4 eV[35],与XRD结果一致。此外,Fe2p能级图中没有观测到卫星峰,这与文献[36,37]中对Fe3O4的测量结果一致,由于Fe(Ⅲ)的卫星结构与光谱中Fe(0)和Fe(Ⅱ)的Fe2p1/2部分重叠,导致无法观测到卫星峰。这进一步说明2种钢在静态及动态水中腐蚀后的外氧化层主要由Fe3O4组成。
图7
图7
9Cr-ODS和CLAM钢在静态和动态高温高压水中腐蚀1000 h后外表面Fe的详细XPS
Fig.7
Detailed XPS of Fe on the outer surface of 9Cr-ODS (a, c) and CLAM (b, d) steels after corrosion in static (a, b) and dynamic (c, d) high temperature and high pressure water for 1000 h
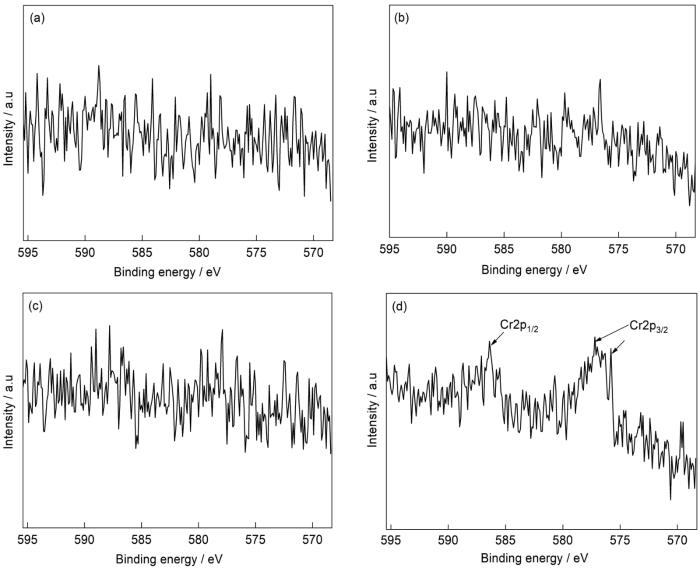
为了确认动态条件下9Cr-ODS钢表面生成Fe2O3,进一步对Fe2p3/2数据进行非线性最小二乘 (NLS)拟合。9Cr-ODS钢在动态条件下腐蚀1000 h后Fe2+和Fe3+的拟合结果如图8所示。Fe3O4相可以表示为FeO·Fe2O3,其Fe2+和Fe3+的标准比值为0.5[38]。然而,由于氧化物颗粒尺寸不均匀以及XPS技术的检测深度有限,导致比值存在一定偏差,如Yamashita和Hayes[36]实际测试得到的Fe3+占比为65%。图8中9Cr-ODS钢表面的Fe3+占比为67.86%,大于文献[36]中的65%。因此,9Cr-ODS钢中的Fe元素在动态条件下除了生成Fe3O4,还以Fe2O3的形式存在,这与XRD结果相符合。
图8
图8
9Cr-ODS钢在动态高温高压水中腐蚀1000 h后Fe2p3/2的非线性最小二乘(NLS)拟合结果
Fig.8
Fe2p3/2 NLS fitting results of 9Cr-ODS steel after corrosion in static high temperature and high pressure water for 1000 h (NLS—non-linear least squares)
图9
图9
9Cr-ODS和CLAM钢在静态和动态高温高压水中腐蚀1000 h后外表面中Cr的详细XPS
Fig.9
Detailed XPS of Cr on the outer surface of 9Cr-ODS (a, c) and CLAM (b, d) steels after corrosion in static (a, b) and dynamic (c, d) high temperature and high pressure water for 1000 h
2.5 截面腐蚀形貌及元素分布
图10为9Cr-ODS和CLAM钢在静态高温高压水中腐蚀不同时间后横截面的SEM像。可见,2种钢的腐蚀层厚度随时间增加而增厚。腐蚀层可明显分为2层:呈颗粒状的外层和呈连续层状的内层。当腐蚀时间不超过500 h时,9Cr-ODS钢腐蚀层厚度增长较快,CLAM钢增长较慢。随时间继续延长至1000 h,增长趋势变得相反,此时,9Cr-ODS钢腐蚀层更薄,约为2.5 μm,而CLAM钢约为4.0 μm。
图10
图10
9Cr-ODS和CLAM钢在静态高温高压水下腐蚀不同时间后横截面的SEM像
Fig.10
Cross-sectional SEM images of corrosion products of 9Cr-ODS (a-c) and CLAM (d-f) steels after corrosion in static high temperature and high pressure water for 200 h (a, d), 500 h (b, e), and 1000 h (c, f)
图11
图11
动态条件下9Cr-ODS钢和CLAM钢中表面腐蚀产物横截面的SEM像
Fig.11
Cross-sectional SEM images showing corrosion product morphologies of 9Cr-ODS (a-c) and CLAM (d-f) steels after corrosion in dynamic high temperature and high pressure water for 200 h (a, d), 500 h (b, e), and 1000 h (c, f)
图12
图12
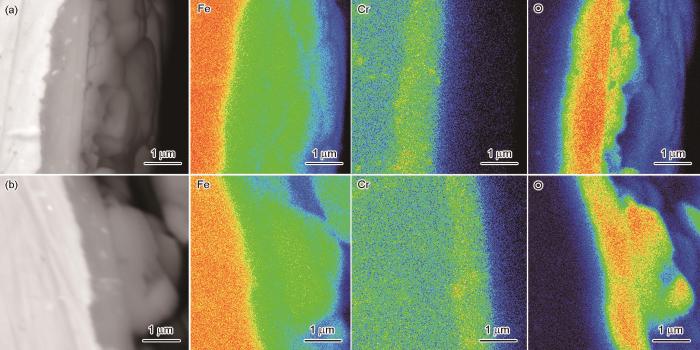
9Cr-ODS钢在静态、动态高温高压水中腐蚀1000 h后横截面的SEM像及EPMA元素分布图
Fig.12
Cross-sectional SEM images and EPMA element maps of 9Cr-ODS steel after corrosion in static (a) and dynamic (b) high temperature and high pressure water for 1000 h
2.6 横截面TEM分析
为了进一步研究9Cr-ODS钢中氧化物的晶体结构,对9Cr-ODS钢在动态高温高压水中腐蚀1000 h后的氧化物进行TEM分析,结果如图13所示。为了使SAED的结果更加准确,选取了较大的氧化物颗粒进行观测,但氧化物颗粒的平均尺寸仍以SEM数据为准。由TEM像(图13a)可知,外氧化物颗粒尺寸约为2.5 μm,内氧化层厚度约为500 nm,内氧化层中晶粒尺寸小于基体中的晶粒尺寸,且基本没有孔隙存在。对氧化膜进行SAED分析,分别选取靠近氧化物外侧的A区域和靠近氧化物中心的B区域,结果如图13b和c所示。经过标定得出,A区域由Fe2O3组成(PDF卡片89-0596),B区域由Fe3O4组成(PDF卡片72-2303),与图4中XRD结果相符。
图13
图13
9Cr-ODS钢在动态高温高压水中腐蚀1000 h后氧化膜截面的TEM像和SAED花样
Fig.13
Cross-sectional TEM image (a) of oxide film of 9Cr-ODS steel after corrosion in dynamic high temperature and high pressure water for 1000 h, and corresponding SAED patterns of zone A (b) and zone B (c) in Fig.13a
3 分析与讨论
3.1 外表面氧化产物
图10~13的分析结果表明,9Cr-ODS和CLAM钢在静态和动态条件下腐蚀1000 h后,氧化层呈现出外部为富Fe的氧化物颗粒、内部为富Cr层的双层结构。根据2种钢的基体元素,利用FactSage软件计算了在325 ℃、25 MPa条件下可能存在的氧化物的标准生成Gibbs自由能,结果由高到低排列,如表5所示。Cr2O3的标准生成Gibbs自由能最低(-672.778 kJ/mol (O2)),是热力学稳定性最高的氧化物,其次为FeCr2O4。然而2种钢的XRD谱中均未检测到Cr2O3的存在,推测其原因为:一方面,2种钢的最高Cr含量仅为9.31%,不足以生成完整的保护性Cr2O3氧化层;另一方面,形成的Cr2O3转化为FeCr2O4,残留的Cr2O3低于XRD的检测极限。因此,2种钢的氧化内层主要为FeCr2O4[39]。计算结果表明,Fe3O4的标准生成Gibbs自由能比Fe2O3更低,因此Fe3O4优先生成,这也是静态和动态水腐蚀实验后外表面检测到Fe3O4的主要原因。Fe3O4转化为Fe2O3时的Gibbs自由能最高,因此反应不易进行。
表5 在325 ℃、25 MPa条件下钢表面氧化物的标准生成Gibbs自由能
Table 5
| No. | Formation | |
|---|---|---|
| 1 | -327.801 | |
| 2 | -446.466 | |
| 3 | -461.299 | |
| 4 | -600.519 | |
| 5 | -654.714 | |
| 6 | -672.778 |
Note: ΔG0—Gibbs free energy, R—gas constant, T—temperature,
3.2 水流状态对耐腐蚀性能的影响
通常采用Wagner[42]理论进行氧化速率预测,理想材料的腐蚀增重曲线一般遵循抛物线增长,但也有研究[43]发现存在偏离抛物线规律的现象。高温高压水氧化动力学可大致分为2类:一类符合抛物线定律(n ≈ 0.500),一类符合立方规律(n ≈ 0.330)。然而,在本工作中,9Cr-ODS钢在动态条件下的n = 0.211,大致符合五次方规律。在理想状态下,Wagner[42]推算腐蚀氧化动力学仅与扩散有关,从而得到抛物线定律。但在实际情况下,增重曲线的n并非总为0.500。Wagner[42]认为,当氧化膜更为疏松时,n在0.500~1.000之间,膜厚度与扩散不按比例增长;而当氧化膜更为致密时,n < 0.500,且致密度越大n越小,这是由于膜中元素扩散系数减小所致。
如图3所示,在静态高温高压水中,2种钢的实验结果更符合立方规律,说明在静态条件下的氧化膜更为致密。然而在动态高温高压水环境中,9Cr-ODS钢的n为0.211,接近于五次方增长,而CLAM钢的n介于抛物线增长(0.500)和立方增长(0.330)之间,说明在动态下9Cr-ODS钢的氧化膜较为致密,而CLAM钢则更为疏松。
与静态相比,动态高温高压水的流动状态的影响主要表现在3个方面:(1) 加速了钢表面的氢氧化物(CrO2(OH)2)溶解,增加了Cr的向外扩散;(2) 减少了在产物表面由于O2分解产生的H2的堆积,提高了氧分压;(3) 增加了对腐蚀产物的冲刷作用。
Asteman等[44]在600 ℃、O2 + 10%H2O/40%H2O条件下对310钢的腐蚀实验表明,在较强的氧化环境下,氧化过程中Cr会生成挥发性的CrO2(OH)2 (g):
在静态条件下,由于氧化反应生成的CrO2(OH)2堆积在界面处,O2及O2-的向内扩散以及Cr3+和Fe2+、Fe3+阳离子的向外扩散被严重阻碍。随流速增加,CrO2(OH)2的挥发量增大,Cr、Fe等元素向外扩散速率增加。当流速低于临界值时,由于金属中Cr3+向外扩散量较小,内部较为致密的Fe-Cr-O尖晶石结构氧化膜仍具有一定的保护性。在静态水中腐蚀至1000 h,对2种钢表面进行XPS分析时均未发现Cr元素的存在,说明Cr未发生明显的向外扩散。在动态水中腐蚀至1000 h时,XPS结果显示,CLAM钢表面有Cr元素存在,说明其FeCr2O4尖晶石结构氧化膜的保护性下降,出现了Cr元素的向外扩散。相反,9Cr-ODS钢表面未检测到Cr的存在,说明其FeCr2O4尖晶石结构氧化膜的保护性优于对比钢。
同时,高温高压水流速的变化,会通过影响表面的O和H分压改变氧化产物,从而影响腐蚀行为。一般在静态水中,由于H2O分解产生的H2堆积在界面处,氢分压增大将会导致表面的O2含量下降,O2和O2-的向内扩散被严重阻碍,从而使得腐蚀速率逐渐降低。Zhang等[30]对P91钢在不同流速下腐蚀行为的研究结果表明,流速通过影响表面H2O分解所产生的H2(g)使得表面的氧分压增大,从而加速了腐蚀的进行并改变了氧化产物类型。Luaszewicz等[45]发现,在T23铁素体钢中,蒸汽流速的增加在增加氧化膜生长速率的同时改变了氧化物相成分,如导致了一层薄内层氧化物的形成。在T92回火马氏体钢中,在较大的蒸汽流速环境下,在氧化膜最外层形成了Fe2O3[45]。因此,本工作中,流动的高温高压水,可通过降低氧化物/腐蚀环境界面处的H2含量,从而增加氧分压,促进了9Cr-ODS钢外表面有Fe2O3的形成。
流速的改变还会影响外表面氧化物颗粒的形貌和数量。一般情况下,流速增大,由于表面的氧分压提高,腐蚀速率增大,腐蚀增重也应相应增加。然而,图3的结果显示,动态条件下2种钢的腐蚀增重反而相较静态下有所降低。图5和6的形貌结果显示,相比静态水条件,2种钢在动态水中表面颗粒的尺寸减小、数量减少。此外,图10和11进一步表明,动态条件下2种钢的腐蚀层厚度相较静态下均有所降低。因此,这种矛盾的合理解释为,流速增大虽然提高了氧化速率,但由于表面Fe3O4层较为稀疏,与基体之间结合力不强,当颗粒生长至一定尺寸后,高温高压水的流动冲刷占主导作用,导致了表面部分颗粒发生剥落,从而使得氧化增重减少以及腐蚀层厚度降低。
3.3 9Cr-ODS钢的耐腐蚀性能
本工作使用的9Cr-ODS钢采用粉末冶金方法制备,与CLAM钢的差异主要为:(1) 由于添加了氧化物,基体中O含量更高;(2) 晶粒尺寸更细,从而使得晶界及元素扩散通道更多;(3) 含有大量弥散分布的纳米氧化物(如Y-Ti-O)。
ODS钢的独特成分与组织会影响腐蚀过程中元素的扩散行为,而氧化产物的生长是由金属阳离子向外扩散和O2-的向内扩散所共同控制[34]。在高温高压水腐蚀条件下,在9Cr系钢铁材料中,一般认为Fe阳离子的向外扩散控制反应进行的速率。
朱忠亮[43]在高温高压水中对P92钢进行18O标定实验后发现,氧化过程中增重的氧源主要来自于水,其与溶解氧对氧化增重贡献的比值约为500∶1;溶解氧对增重的影响主要在于提高了氧化膜内的氧势梯度,进而加速了阳离子在氧化膜内的扩散速率。
基于Wagner[46]的氧化理论,氧化速率常数可表示为:
式中,k为速率常数;
式中,
由于磁铁矿和尖晶石结构具有较为相似的晶体结构,铁离子在2种氧化物中的自扩散特性较为相似,将
因此,氧化膜外侧与内侧界面的氧分压定义如下:(1) 氧化层/腐蚀环境界面的氧分压与腐蚀环境中的溶解氧有关;(2) 氧化膜/基体界面的氧分压与基体的O含量、O扩散速率以及尖晶石分解平衡压有关。
9Cr-ODS钢中由于加入了Y2O3氧化物颗粒,在基体中引入了更高的O含量,影响了
此外,9Cr-ODS钢的晶界中还存在大量稳定性高的Y-Ti-O氧化物颗粒。已有研究[23]证明,这些弥散分布的氧化物颗粒在腐蚀过程中可阻碍和吸附离子的扩散,从而阻碍腐蚀反应的进一步进行,提高了钢的耐腐蚀性能。
3.4 腐蚀机制
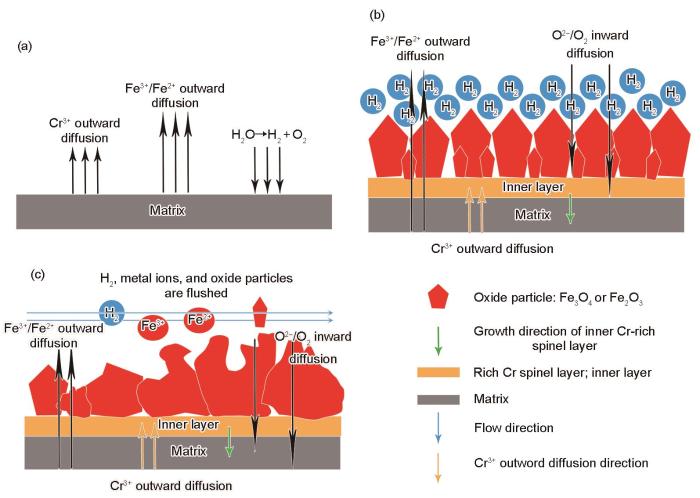
综上所述,9Cr-ODS钢在325 ℃、15.5 MPa的静态、动态高温高压水条件下的腐蚀过程示意图如图14所示。如图14a和b所示,在静态高温高压水中,腐蚀初期金属与水直接接触,高温高压水在金属表面被分解为H2和O2,基体中的Fe、Cr被氧化成Fe3+、Fe2+、Cr3+,由于Fe3+、Fe2+的扩散速率大于Cr3+,通过固态传输[50,51]以及溶解-沉淀机制[52]优先形成规则的Fe3O4颗粒,并在基体中生成FeCr2O4。随着腐蚀的进一步进行,H2O分解所产生的H2无法被消耗,堆积在金属表面,阻碍了O2的生成和扩散,腐蚀速率放缓,此时外表面氧化物颗粒保持完整。随着腐蚀时间进一步增加,氧化物颗粒的直径增大,腐蚀内层的厚度增加。如图14c所示,在动态高温高压水中,由于水流状态的改变,将使得H2无法在表面堆积。腐蚀初期在金属表面生成了更多的氧化物颗粒,并提高了腐蚀的反应速率[30]。随着反应的进行,氧化物颗粒生长到较大尺寸,在水流冲蚀下,外层氧化物存在着氧化物颗粒剥落的情况,同时氧化物产物类型也发生改变。此时氧化产物从外向内依次为Fe2O3、Fe3O4、FeCr2O4。规则的氧化物颗粒形态同样发生改变,转变为类似絮状的产物。
图14
图14
9Cr-ODS钢在静态、动态高温高压水中腐蚀过程的示意图
Fig.14
Schematics of corrosion process of 9Cr-ODS steel in static and dynamic high temperature and high pressure water
(a) early stage of corrosion (b) static (c) dynamic
4 结论
(1) 9Cr-ODS钢在静态条件下的腐蚀增重遵循立方规律,而在动态下的腐蚀增重遵循五次方规律。与CLAM钢相比,9Cr-ODS钢在静态与动态条件下腐蚀增重均较低。
(2) 在静态条件下,9Cr-ODS钢外表面覆盖有形状规整的氧化物颗粒,并随时间延长氧化物颗粒尺寸逐渐长大;腐蚀至1000 h后,氧化膜总厚度大约为2.5 μm。在动态条件下,外表面氧化物颗粒尺寸减小,出现絮状产物,数量降低,1000 h时的氧化膜总厚度约为1.68 μm。
(3) 在静态条件下,2种钢均形成外层为Fe3O4、内层为富Cr氧化物层的双层氧化膜结构。在动态条件下,9Cr-ODS钢外层为Fe3O4和少量Fe2O3,内层为FeCr2O4;而CLAM钢的外层为Fe3O4,内层为FeCr2O4。
(4) 高温高压水的流动状态改变了表面的氢和氧分压以及H2O的冲刷作用,从而影响了表面氧化物的形成行为和腐蚀增重。
(5) 9Cr-ODS钢中细小的晶粒尺寸、较高的晶界数量和O含量以及沿晶界分布的纳米氧化物等特性是其具有更低腐蚀速率的主要原因。
参考文献
Primary analysis of radiation damage on first wall and the outboard blanket on equatorial plane for CFETR
[J].
CFETR第一壁及赤道面外包层中子辐照损伤初步分析
[J].
Overview of the present progress and activities on the CFETR
[J].
Microstructural investigation of the breakdown of the protective oxide scale on a 304 steel in the presence of oxygen and water vapour at 600 oC
[J].
Continuous and cyclic oxidation of T91 ferritic steel under steam
[J].
Influence of alloy composition and temperature on corrosion behavior of ODS ferritic steels
[J].
Some studies on P91 steel and their weldments
[J].
Effect of precipitates on long-term creep deformation properties of P92 and P122 type advanced ferritic steels for USC power plants
[J].
High-temperature strength characterization of advanced 9Cr-ODS ferritic steels
[J].
Low cycle fatigue behavior of 9Cr-ODS Steel as a fusion blanket structural material at room temperature
[J].Adequate studies have not been conducted on the low cycle fatigue properties of oxide dispersion strengthened (ODS) steels that are typically used for fusion reactors worldwide. Moreover, the majority of the fatigue properties are examined with a small sample size due to the restricted manufacturing capacity, which is insufficient for determining the comprehensive properties of bulk materials. Research on the fatigue properties of Chinese ODS steels was conducted recently. However, it is quite uncommon to report the fatigue properties of self-produced 9Cr-ODS steel. Consequently, this work is the first to examine the effect of a cyclic strain on the low cycle fatigue behavior of a representative low-activation 9Cr-ODS martensitic steel. Hence, the strain control test method was employed here in a strain amplitude range of 0.3%-0.8% at room temperature. The cyclic stress response curves, hysteresis loops, relationships between strain amplitude and life, stress amplitude and plastic strain were obtained, and the corresponding fatigue parameters were summarized. Furthermore, the microstructural evolution, fatigue fracture morphology, and crack propagation characteristics during the fatigue process were analyzed. The results revealed that the cyclic stress response behavior of the 9Cr-ODS steel was related to the strain amplitude. With an increase in the strain amplitude, the peak stress in the tension zone of 9Cr-ODS steel increased and the fatigue life decreased. The relationship between cyclic strain and life agreed well with the Coffin-Manson model. Additionally, the 9Cr-ODS steel had no obvious cyclic hardening but revealed a cyclic softening under higher strain amplitudes of 0.5%-0.8%. The microstructure analysis showed that for higher cyclic strain amplitudes, the average grain size and the fraction of the large-angle grain boundaries increased gradually with a reduction in the dislocation density, leading to the cyclic softening of the material. The fatigue crack initiated at the surface and propagated inward by the transgranular mode. The fine grain boundaries and subgrain boundaries of the 9Cr-ODS steel could induce crack deflection, reduce crack propagation rate, and increase fatigue crack propagation life. Moreover, under the same strain amplitude, the peak stress in the tension zone of the steel was almost twice that of the China low activation martensitic (CLAM) steel without a reduction in the fatigue life, indicating a superior low cycle fatigue resistance of the 9Cr-ODS steel.
聚变增殖包层用低活化9Cr-ODS钢的室温低周疲劳行为
[J].在现有研究中,低活化9Cr-ODS钢虽然具有优异的高温性能,但其低周疲劳性能及微观组织演变规律尚未全面揭示,本工作旨在揭示其在不同应变幅下的循环响应和裂纹扩展特性。采用应变控制测试方法,研究了低活化9Cr-ODS钢在0.3%~0.8%应变幅范围内的室温低周疲劳性能,包括循环应力响应曲线、滞后回线、应变幅与寿命以及应力幅与塑性应变的关系,给出了相应的疲劳参数,并分析了疲劳过程中微观组织演变、疲劳断口形貌及裂纹扩展特性等。结果表明,9Cr-ODS钢的循环应力响应行为与应变幅有关。随应变幅增加,材料的峰值拉应力增加,疲劳寿命降低,循环应变-寿命满足Coffin-Manson关系。9Cr-ODS钢在所有应变幅下未出现明显的循环硬化,但在0.5%~0.8%较高应变幅范围内发生了一定的循环软化。微观组织分析表明,随循环应变幅增加,亚结构发生了回复,位错密度降低,平均晶粒尺寸及大角度晶界占比逐渐增加,从而导致9Cr-ODS钢的循环软化。疲劳裂纹起源于样品表面,但裂纹源附近均未发现明显的夹杂物或析出相。裂纹以穿晶方式扩展,9Cr-ODS钢细的原始奥氏体晶界及亚晶界对裂纹扩展具有强烈的抑制作用。此外,在相同低应变幅且不降低循环疲劳寿命条件下,9Cr-ODS钢所能承受的峰值拉应力达到低活化铁素体/马氏体钢的2倍,显示出较优越的低周疲劳强度。
Microstructure and mechanical properties of a novel designed 9Cr-ODS steel synergically strengthened by nano precipitates
[J].Oxide dispersion-strengthened (ODS) steels with nano-scale Y2O3 or Y-Ti-O oxides have been considered as potential structural materials used in advanced nuclear systems. In this work, a novel 9Cr-ODS steel, namely, MX-ODS steel, was designed by decreasing carbon content to eliminate conventional M23C6-type carbides and by increasing the content of nitrogen and vanadium to form MX-type precipitates. In addition, the MX-ODS steel was synergistically strengthened by nano-scale MX precipitates and oxides. After fabrication by powder metallurgy, microstructural observation, and mechanical property tests were conducted after different heat treatments. The density of the prepared materials using hot forging instead of hot isostatic pressing was about 98%. Results of the microstructure observation of the MX-ODS steel indicated that after normalizing and tempering, the tempered martensitic structure dominated, and the mean effective grain size was approximately 1 μm. Moreover, the preferential orientation of coarse-grained and fine-grained mixed grains was not detected. By diminishing carbon content, M23C6-type carbides precipitated at the grain and sub-grain boundaries were eliminated. By contrast, MX-type precipitates with a diameter of approximately 30-200 nm were formed in the matrix. Furthermore, nano-scale Y-rich oxides with an average size of approximately 3.0 nm were dispersed in the matrix, and a number density can reach to approximately 1.9 × 1023 m-3. Furthermore, “core-shell” structure precipitates were found, which were spherical in shape with a diameter ranging from 10 to 20 nm. Such precipitates also contained Y, Ta, and O as the core and V as the shell. The mechanical properties indicate that microhardness decreased from 372 to 320 HV with the increase of normalizing temperature from 980oC to 1200oC. In addition, microhardness decreased significantly after tempering but initially increased and then decreased with the increase of tempering temperature from 700oC to 800oC, with a peak microhardness at approximately 750oC. Excellent strength and ductility were obtained after normalizing at 1100oC for 1 h and then tempering at 750oC for 1 h. Yield strength, ultimate tensile strength, and total elongation were 1039 MPa, 1103 MPa, and 20.5% when tested at room temperature and 291 MPa, 333 MPa, and 16% at 700oC, respectively.
新型纳米复合强化9Cr-ODS钢的设计、组织与力学性能
[J].
On the thermal stability of a 9Cr-ODS steel aged at 700 oC up to 10000 h—Mechanical properties and microstructure
[J].
Grain structural characterization of 9Cr-ODS steel aged at 973 K up to 10,000 h by electron backscatter diffraction
[J].
Study on the formation mechanism of Y-Ti-O oxides during mechanical milling and annealing treatment
[J].
Corrosion and stress corrosion cracking in supercritical water
[J].
Microstructure characteristic and mechanical property of transformable 9Cr-ODS steel fabricated by spark plasma sintering
[J].
Corrosion of ODS steels in lead-bismuth eutectic
[J].
Corrosion behaviors and mechanisms of ODS steel exposed to static Pb-Bi eutectic at 600 and 700 oC
[J].
ODS钢在600和700 ℃静态Pb-Bi共晶中的腐蚀行为及机理
[J].以一种极具潜力的先进核能候选氧化物弥散强化(ODS)钢为研究对象,以不控制氧浓度的液态金属Pb-Bi共晶(LBE)为腐蚀介质,研究了静态下高温(600和700 ℃)不同腐蚀时间对ODS钢腐蚀行为的影响及其微观机制。结果表明,600 ℃腐蚀至2000 h,ODS钢表面生成总厚度约为10 μm的典型双层氧化膜,同时在内氧化层下方形成了一层较薄的富Al氧化层。基于致密的尖晶石内氧化层及富Al氧化层的保护作用有效减缓了腐蚀氧化速率,ODS钢显示优异于其它材料的耐LBE腐蚀性能。ODS钢在700 ℃腐蚀所形成的氧化膜结构及厚度与600 ℃明显不同:腐蚀100 h主要形成了厚度约为500 nm的Al<sub>2</sub>O<sub>3</sub>保护膜,大幅降低了腐蚀速率;腐蚀时间延长至500 h,大部分区域Al<sub>2</sub>O<sub>3</sub>氧化膜仍然存在,但同时出现的少量“疖状氧化物”破坏了Al<sub>2</sub>O<sub>3</sub>保护膜的连续性,从而成为了腐蚀的突破口。
Corrosion behavior of 9Cr-ODS steel in stagnant liquid lithium and lead-lithium at 873 K
[J].
The effect of surface grinding and Si addition on the corrosion of Fe-12Cr ODS steels in supercritical CO2
[J].
A study of the oxidation behaviour of FeCrAl-ODS in air and steam environments up to 1400 oC
[J].
Corrosion behavior of 14Cr ODS steel in supercritical water: The influence of substituting Y2O3 with Y2Ti2O7 nanoparticles
[J].
Mechanistic study of incipient corrosion for nuclear grade lean-Cr FeCrAl alloys in a simulated PWR environment
[J].
Role of Al addition and Y2O3 on the intergranular corrosion behavior of AFA-ODS steel in the supercritical water
[J].
Corrosion behavior of ferritic ODS steel prepared by adding YH2 nanoparticles in supercritical water at 600 oC
[J].
Oxidation behavior of 9Cr-4.5Al ODS steel in 600 oC supercritical water and the effect of pre-oxidation
[J].
Oxidation behavior of iron-based alloy HCM12A exposed in supercritical water
[J].
Corrosion behavior of F82H exposed to high temperature pressurized water with a rotating apparatus
[J].
Effects of heat treatment on microstructure and mechanical properties of a bimodal grain ultra-low carbon 9Cr-ODS steel
[J].Oxide dispersion strengthened (ODS) steel is a promising structural material for advanced nuclear power systems. In this study, an ultra-low carbon 9Cr-ODS steel with a bimodal grain structure was prepared using powder metallurgy, and a superior matching of strength and plasticity was expected by adjusting the soft-hard matching of the coarse-grained and fine-grained regions. The effects of heat treatment on microstructure and mechanical properties of the ultra-low carbon 9Cr-ODS steel were evaluated through OM, SEM, TEM, microhardness, and tensile tests. The results demonstrated that the ultra-low carbon 9Cr-ODS steel exhibited a tempered martensite structure after normalizing at 1050-1200oC, and then tempering at 700 and 750oC. Moreover, it presented the microstructure characteristics of coarse-grained and fine-grained regions, in which the average grain size of fine-grained regions was 1.6 μm and that of coarse-grained regions was 4.3 μm. The dislocation density in the ultra-low carbon 9Cr-ODS steel was very high and the number density of nano-scale oxide particles was up to about 1022 m-3. The microhardness in fine-grained regions was higher than that in coarse-grained regions. As the normalizing temperature increased, the microhardness of the ultra-low carbon 9Cr-ODS steel first increased and then decreased. The microhardness reached the highest after normalizing at 1100oC. When the normalizing temperature increased to 1200oC, the microhardness decreased due to the growth of austenitic grains. Regarding the tempering temperature, the microhardness first decreased and then increased as the tempering temperature increased from 700oC to 800oC. Furthermore, the decrease in microhardness when tempering at 700 and 750oC was because the microstructure was recovered and softened. The higher the tempering temperature, the lower the microhardness. However, when tempering at 800oC, the microhardness increased significantly, mainly due to the partial austenite transformation of martensite. The tensile test results at 25oC showed that the strength of the ultra-low carbon 9Cr-ODS steel first decreased and then increased by increasing the tempering temperature, which was consistent with the microhardness change while the opposite was observed for elongation. The tensile test results at 700oC showed that the strength of the ultra-low carbon 9Cr-ODS steel slightly decreased by increasing the tempering temperature. Moreover, the fracture morphology was dominated by fine dimples and secondary tearing, indicating that the ultra-low carbon 9Cr-ODS steel underwent ductile fracture. Combined with the mechanical property and fracture analysis results, the ultra-low carbon 9Cr-ODS steel exhibited superior matching of strength and plasticity after normalizing at 1150oC for 1 h and tempering at 750oC for 1 h.
热处理对一种双峰晶粒结构超低碳9Cr-ODS钢显微组织与力学性能的影响
[J].采用粉末冶金方法制备了一种具有双峰晶粒结构的超低碳9Cr-ODS (氧化物弥散强化)钢,通过OM、SEM、TEM、显微硬度和拉伸性能测试,研究了热处理工艺对其显微组织和力学性能的影响。结果表明,超低碳9Cr-ODS钢经正火+回火后为回火马氏体组织,具有粗、细晶分明的结构特征。细晶区的平均晶粒尺寸约为1.6 μm,粗晶区的平均晶粒尺寸约为4.3 μm。同时,基体中存在大量的位错结构,且纳米级氧化物数密度可达约10<sup>22</sup> m<sup>-3</sup>。不同的热处理工艺不会改变超低碳9Cr-ODS钢粗、细晶双峰晶粒结构特征。经热处理后,细晶区比粗晶区具有更高的硬度。随正火温度升高,粗、细晶区的显微硬度先上升后下降,且在1100℃正火时达到最高。正火温度一定,回火温度从700℃升高至800℃时,粗、细晶区的显微硬度先下降后上升。700和750℃回火时,组织得到回复,发生软化,温度越高硬度越低;而在800℃回火时,超低碳9Cr-ODS钢因发生部分奥氏体相变导致硬度提高。25℃拉伸实验结果与硬度的变化趋势一致,随回火温度升高,超低碳9Cr-ODS钢的强度先降低后增加,延伸率则呈现相反趋势。700℃拉伸实验结果表明,超低碳9Cr-ODS钢的强度随回火温度的升高稍有降低。结合力学性能及断口分析结果,分析了双峰晶粒结构超低碳9Cr-ODS钢的断裂机制。经1150℃、1 h正火+ 750℃、1 h回火后,超低碳9Cr-ODS钢具有最优的强塑性匹配。
Effect of heat treatment processes on microstructure and mechanical properties of ton-scale china low activation martensitic steel
[J].
热处理工艺对吨级CLAM钢组织及力学性能的影响
[J].分析了正回火热处理制度对吨级中国低活化马氏体(CLAM)钢的组织与力学性能的影响。结果表明,正火温度主要影响吨级CLAM钢马氏体组织中原奥氏体晶粒度的大小,回火温度主要影响组织中的亚结构;相比正火温度,回火温度对吨级CLAM钢力学性能的影响较大。吨级CLAM钢在980 ℃正火+760 ℃回火的热处理条件下可获得最佳的组织与综合力学性能。
Oxidation of ferritic and ferritic-martensitic steels in flowing and static supercritical water
[J].
Oxidation behavior analysis of a ferritic ODS steel in supercritical Water
[J].
Corrosion behavior of a 14Cr-ODS steel in supercritical water
[J].
Ferritic-martensitic steels for fission and fusion applications
[J].Compared to austenitic stainless steels, largely employed in the early fission reactors, high chromium Ferritic/Martenstic (FM) steels, developed since the first half of the 20th century for fossil-fuel power-plants, have a number of advantageous properties among which lower thermal expansion, higher thermal conductivity and better void swelling resistance. At the beginning of the 1970s, FM steels found their first nuclear application as wrapper and fuel cladding materials in sodium-cooled fast reactors. They are now the reference materials for in-vessel components of future fusion reactors, and are considered for in-pile and out-of-pile applications in Generation IV reactors as well as for various other nuclear systems. In this paper, after an introductory historical overview, the challenges associated with the use of FM steels in advanced reactors are addressed, including fabrication, joining and codification issues. The long term evolution of mechanical properties such as the creep and creep-fatigue behaviors is discussed and the degradation phenomena occurring in aggressive environments (lead alloys, high temperature gases, super-critical water and CO2, molten salts) are detailed. The paper also provides a brief overview of the radiation effects in FM steels. The influence of the key radiation parameters e.g. temperature, dose and dose rate on the microstructure and mechanical properties are discussed. The need to better understand the synergistic effects of displacement damage and helium produced by transmutation in fusion conditions is highlighted. (C) 2019 Elsevier B.V.
Corrosion behavior of 9-12% Cr ferritic-martensitic steels in supercritical water
[J].
Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials
[J].
Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni
[J].
Corrosion behavior of Fe-Cr-Si alloys in simulated PWR primary water environment
[J].
A review of the oxidation behaviour of structural alloys in steam
[J].
Corrosion behavior of ferritic-martensitic steel T91 in supercritical water
[J].
Corrosion of ferritic-martensitic steel HT9 in supercritical water
[J].
Oxidation of 310 steel in H2O/O2 mixtures at 600 oC: The effect of water-vapour-enhanced chromium evaporation
[J].
Effect of steam flow rate and sample orientation on steam oxidation of ferritic and austenitic steels at 650 and 700 oC
[J].
The distribution of cations in metal oxide and metal sulphide solid solutions formed during the oxidation of alloys
[J].
Porosity prediction in supercritical water exposed ferritic/martensitic steel HCM12A
[J].
Effect of alloy grain size and silicon content on the oxidation of austenitic Fe-Cr-Ni-Mn-Si alloys in pure O2
[J].
Grain-size effects on the high-temperature oxidation behaviour of chromium steels
[J].
The mechanism of high temperature aqueous corrosion of stainless steels
[J].
Early corrosion behaviour of irradiated FeCrAl alloy in a simulated pressurized water reactor environment
[J].
The mechanism and kinetics of corrosion product release from stainless steel in lithiated high temperature water
[J].