微生物腐蚀(microbiologically influenced corrosion,MIC)是指由于微生物活动引起、促进或加速材料腐蚀的现象[1]。它可广泛发生于各种工业设施中,包括供水系统[2]、地下储罐[3]、船舶[4]、油气管道等。据统计,有超过20%的管道系统故障与MIC密切相关[5]。文献中报道了多起微生物腐蚀导致的管道失效事故。2015年10月底至2016年2月,美国加州洛杉矶郊外的Aliso Canyon地下天然气储存设施管道失效造成泄露,在此期间超过105 t甲烷释放到大气中,造成了巨大的气候影响和经济损失[6]。事后调查认为地下水中的产甲烷菌引起的外部腐蚀是本次事故的罪魁祸首。2007年BP石油公司位于阿拉斯加Prudhoe湾的原油管道由于硫酸盐还原菌(sulfate reducing bacteria,SRB)的腐蚀而发生内部穿孔,导致了7.5 × 105 L的原油泄漏,造成严重环境污染[7]。
近年来,研究者从材料的角度出发,利用Cu元素的抗菌性能,开发出具有耐微生物腐蚀性能的管线钢。Yu等[14]开发了Cu含量为0.6% (质量分数,下同)的L245管线钢,其在实际工况条件的实验中表现出了良好的耐蚀性,然而其表面仍然发生了明显的点蚀;史显波等[15,16]开发出Cu含量为1%~2%的新型X80级管线钢,研究结果表明Cu含量的提高可以提高钢的抗菌性能;然而当Cu含量从1.0%提高到2.0%时,钢在-20℃的冲击功降低了2/3。此外,过高的Cu含量还会增加材料的成本,引起管线钢在热加工过程中出现“热脆”等问题,严重影响了含Cu管线钢的实际应用[17,18]。因此,为平衡含Cu耐微生物腐蚀管线钢的力学性能、耐微生物腐蚀性能和生产成本,亟待对其化学成分、组织等进行优化。
本工作着眼于含Cu管线钢的耐微生物腐蚀性能,设计了不同Cu含量的X65级管线钢,采用电化学阻抗谱(EIS)、线性极化电阻(LPR)、动电位极化曲线等电化学测试手段,结合扫描电镜(SEM)、激光共聚焦显微镜(CLSM)、X射线光电子谱仪(XPS)等形貌表征和成分分析手段,研究了钢中Cu含量对其耐蚀性能的影响,为耐微生物腐蚀管线钢的优化设计提供依据。
1 实验材料与方法
1.1 实验材料与溶液
实验材料成分及编号如表1所示。1#实验用钢使用25 kg真空感应炉冶炼,铸锭经锻造、轧制制成钢板,随后对部分轧制钢板进行500℃、1 h的时效处理。2#、3#实验用钢是在高纯度Ar气气氛中,采用电弧熔炼纯金属(纯度99.9%)的方法制备;为保证实验用钢的成分均匀,将其反复熔炼4次,浇注在尺寸为50 mm × 15 mm × 3 mm的模具中。对铸锭在1150℃下均匀化处理10 min,然后进行冷轧,变形率为67%。冷轧后的钢板在900℃固溶处理30 min后空冷至室温,随后进行500℃、1 h的时效处理。所有样品从时效处理后的轧板切取。显微组织观察和电化学实验样品从3种轧板上切取,尺寸为10 mm × 10 mm × 3 mm。对于电化学实验样品,将样品去除氧化皮,逐级打磨至800号,用蒸馏水、无水乙醇清洗后烘干,之后在样品背面焊接上导线,并将除工作面外的部分用环氧树脂封装,工作面积为10 mm × 10 mm。样品使用前用紫外灯灭菌30 min。
表1 实验用钢的成分 (mass fraction / %)
Table 1
| Steel | C | Si | Mn | Cu | Nb | Ti | Mo | Fe |
|---|---|---|---|---|---|---|---|---|
| 1# | 0.02 | 0.01 | 0.07 | 1.34 | 0.04 | 0.017 | 0.1 | Bal. |
| 2# | 0.02 | 0.01 | 0.05 | 0.70 | 0.04 | 0.017 | 0.1 | Bal. |
| 3# | 0.02 | 0.01 | 0.05 | - | 0.04 | 0.017 | 0.1 | Bal. |
实验中所用的SRB菌株分离自国家材料环境腐蚀试验站沈阳土壤中心站,采用API-RP38培养基对SRB进行富集培养。API-RP38培养基成分为:NaCl 10.0 g/L,MgSO4·7H2O 0.2 g/L,乳酸钠4.0 g/L,酵母膏1.0 g/L, K2HPO4 0.5 g/L,抗坏血酸0.1 g/L,(NH4)2Fe(SO4)2 0.02 g/L,用5 mol/L的NaOH调节pH值至7.0~7.2之间。富集后的菌种保存在4℃环境中,实验前菌种在30℃恒温箱活化12 h。
实验所用的测试溶液为灭菌(NS)和接种SRB的近中性土壤模拟液(NS4溶液),其化学组成为:NaHCO3 0.483 g/L,KCl 0.122 g/L,CaCl2 0.137 g/L,MgSO4·7H2O 0.131 g/L。溶液采用去离子水和分析纯浓度的药剂配制。溶液配制完成后,向溶液中通入体积比为5%CO2 + 95%N2的混合气体2 h除氧,然后在121℃下高压灭菌20 min后储存备用。对于有菌体系,实验前在灭菌NS4溶液加入体积分数为5%的活化后的SRB菌种;对于无菌对照组,加入相同体积的无菌培养基。
1.2 实验方法
组织观察样品经机械研磨并抛光后,用2%硝酸酒精腐蚀以显示组织,使用LSM 710型CLSM观察轧板金相组织。电化学测试在PARSTAT 2273测试系统上进行。实验在30℃恒温水浴锅中进行,周期为14 d,实验溶液为无菌和接菌的NS4溶液。测试采用经典的三电极体系,工作电极为不同Cu含量的管线钢,辅助电极为Pt片,参比电极使用饱和甘汞电极(SCE)。记录实验过程中工作电极的开路电位(EOCP)、线性极化电阻(
使用LSM 710型CLSM表征样品的抗菌性能。将在有菌NS4溶液中浸泡14 d后的样品取出,用磷酸盐缓冲溶液(PBS)洗去未附着的细菌,将样品浸泡在一定浓度的核酸荧光染料(LIVE/DEADTM BacLightTM Bacterial Viability Kit,美国)中,其中,SYTO 9用于染活细菌,PI用于染死细菌,避光染色15 min后,用PBS缓冲液冲洗浮色。用CLSM观察样品表面活/死细菌分布,活细菌呈现绿色,死细菌呈现红色。
实验结束后,对于有菌溶液的浸泡样品,取出后首先在含有3%戊二醛的PBS溶液中浸泡2 h以固定生物被膜,然后依次用50%、75%、90%、100% (体积分数)的乙醇溶液逐级脱水,每次10 min脱水结束后迅速吹干;对无菌溶液浸泡的样品,使用无水乙醇清洗并迅速吹干。随后在处理后的样品表面喷金。使用JSM-6301 SEM观察样品表面的腐蚀产物形貌,并通过SEM自带的能谱仪(EDS)对试样表面产物进行元素分析。完成腐蚀产物分析后,将样品浸入除锈剂(500 mL盐酸 + 500 mL去离子水 + 20 g六次甲基四胺)中去除腐蚀产物,并用去离子水清洗吹干,在SEM下观察腐蚀形貌。利用LSM700 CLSM观察并统计样品表面的点蚀坑数量、尺寸及深度。
采用ESCALAB 250型XPS分析腐蚀产物中Cu元素的化学状态,实验结果通过标准C谱(284.8 eV)校准,并使用XPS PEAK软件进行数据处理。
2 实验结果
2.1 显微组织
不同Cu含量管线钢的显微组织如图1所示。可见,3种管线钢的显微组织均为多边形铁素体组织,但是其晶粒尺寸不同,1#、2#、3#钢的平均晶粒尺寸分别为24、13和18 μm。
图1
图1
不同Cu含量管线钢的显微组织
Fig.1
Microstructures of Cu-bearing pipeline steels with different Cu contents
(a) 1# (b) 2# (c) 3#
2.2 电化学测试结果
2.2.1 OCP、LPR和动电位极化曲线
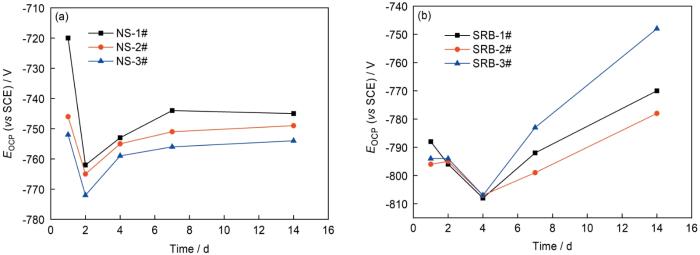
图2是3种实验用钢在无菌及有菌NS4溶液中浸泡14 d的EOCP随时间的变化规律。3种实验用钢在无菌及接菌溶液中的变化趋势相似。在无菌溶液中,1#钢的EOCP在第2 d下降了约50 mV,2#及3#钢下降了约20 mV;在第2~7 d内,1#、2#、3#钢的EOCP均上升了20 mV,之后基本保持不变,且随着Cu含量的降低而逐渐下降。在有菌溶液中,其EOCP较无菌溶液更低。3种钢的EOCP均在1~4 d下降,之后逐渐升高。EOCP的下降,可能是由于SRB及其形成的生物膜在实验用钢表面上附着导致的。其次,细菌的增殖改变了溶液的组分,生成的硫化物使溶液的导电性增加[19];而之后EOCP的升高,是由于腐蚀产物在样品表面上积累导致的。开路电位反映了材料腐蚀的热力学倾向,从无菌和接菌的实验结果可以看出,随着Cu含量的降低,实验用钢腐蚀的热力学倾向增高。
图2
图2
灭菌和接菌NS4溶液中不同Cu含量管线钢的开路电位随时间的变化
Fig.2
Open circuit potential (EOCP) of Cu-bearing steels with different Cu contents in sterile (a) and SRB inoculated (b) NS4 solutions as a function of time (SRB—sulfate reducing bacteria, NS—no SRB, SCE—saturated calomel electrode)
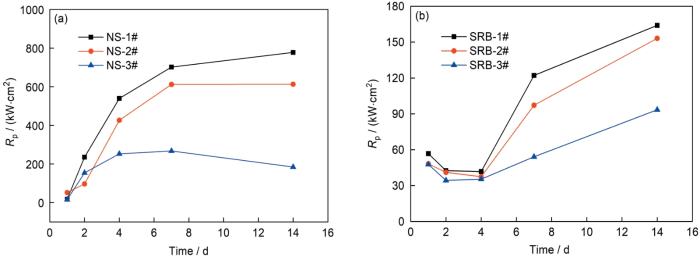
一般而言,可用
图3
图3
不同Cu含量管线钢在灭菌和接菌NS4溶液的线性极化电阻(
Fig.3
Time dependence of
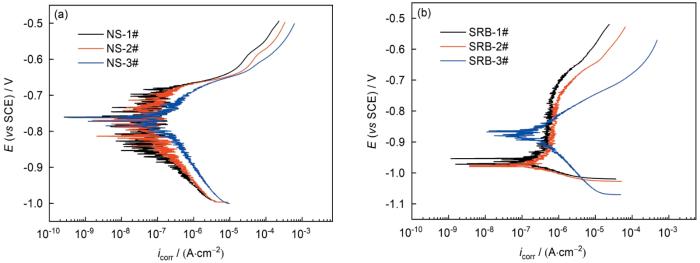
图4是样品在无菌及有菌环境中浸泡14 d后的动电位极化曲线。在无菌环境中,3种钢均表现出活性溶解的特征,Cu的加入同时抑制了阳极和阴极反应;采用Tafel外推法对1#、2#、3#钢的动电位极化曲线进行拟合,所得腐蚀电流密度分别为(0.486 ± 0.120)、(0.673 ± 0.204)和(1.045 ± 0.193) μA/cm2,可见钢的腐蚀速率随着Cu含量的提高而降低。在有菌环境中,1#和2#钢呈现出一定的扩散控制的特征,3#钢仍为活性溶解,3种钢拟合所得腐蚀电流密度分别为(0.717 ± 0.306)、(0.938 ± 0.272)和(2.031 ± 0.414) μA/cm2。可以看出,有菌环境中,3种实验用钢试样的腐蚀速率同样随着Cu含量的提高而降低。
图4
图4
不同Cu含量管线钢在无菌和接菌NS4溶液中浸泡14 d后的动电位极化曲线
Fig.4
Potentiodynamic polarization curves of Cu-bearing steels with different Cu contents immersed in sterile (a) and SRB inoculated (b) NS4 solutions for 14 d (E—potential, icorr—corrosion current density)
2.2.2 EIS
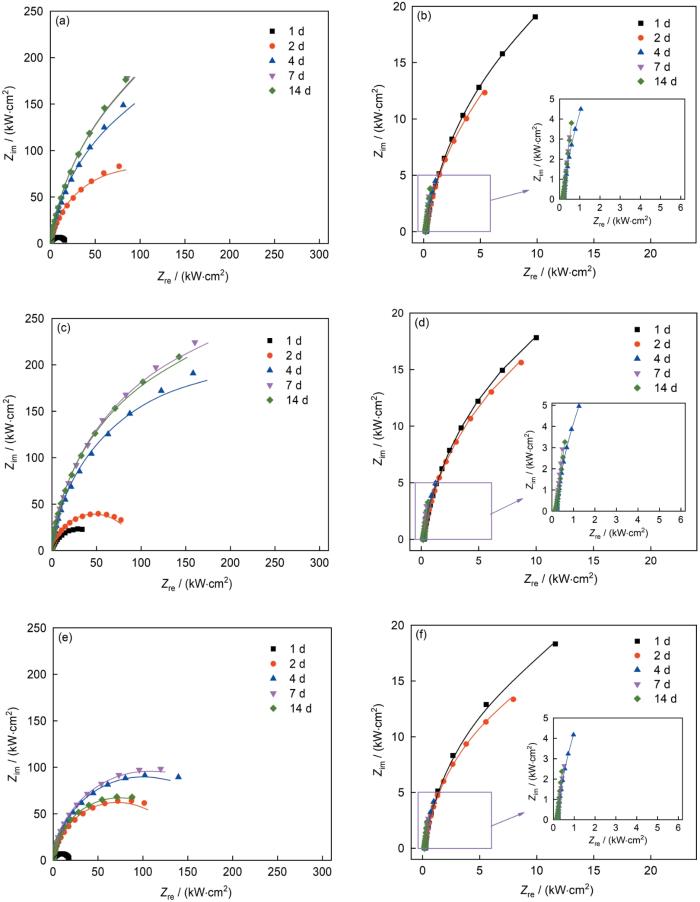
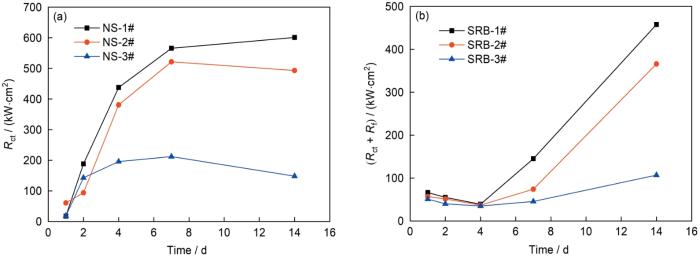
图5为无菌和接菌NS4溶液中3种实验用钢的EIS结果。从Nyquist图可以看到,无菌环境中,阻抗谱表现为一个时间常数。实验前7 d,随着浸泡时间的延长,阻抗半径不断增大,说明钢的腐蚀速率不断减小;7 d后阻抗半径基本不变,说明钢的腐蚀速率不再变化。在有菌环境中,阻抗半径呈现先减小(1~4 d)再增大(4~14 d)的趋势,说明钢的腐蚀速率呈现先增大再减小的趋势。
图5
图5
不同Cu含量管线钢在无菌和接菌NS4溶液中的Nyquist谱随时间的变化
Fig.5
Nyquist plots of Cu-bearing steels with different Cu contents during 14 d immersion in sterile (a, c, e) and SRB inoculated (b, d, f) NS4 solutions
(a, b) 1# (c, d) 2# (e, f) 3#
图6
图6
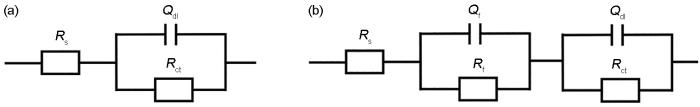
用于EIS拟合的等效电路模型
Fig.6
Equivalent circuits model used to fit EIS data (
(a) equivalent circuit for one-time constant (b) equivalent circuit for two-time constant
表2 灭菌NS4溶液中的EIS拟合结果
Table 2
| Steel | Time / d | Rs / (Ω·cm2) | Qdl | Rct / (Ω·cm2) | ||
|---|---|---|---|---|---|---|
| Ydl / (S·s n ·cm-2) | ndl | |||||
| NS-1# | 1 | 193.6 | 1.387 × 10-4 | 0.8365 | 1.733 × 104 | 6.07 × 10-4 |
| 2 | 188.8 | 7.166 × 10-5 | 0.8953 | 1.881 × 105 | 8.79 × 10-4 | |
| 4 | 175.8 | 5.981 × 10-5 | 0.9082 | 4.377 × 105 | 7.93 × 10-4 | |
| 7 | 169.2 | 5.488 × 10-5 | 0.9110 | 5.946 × 105 | 8.95 × 10-4 | |
| 14 | 141.9 | 5.519 × 10-5 | 0.9104 | 6.011 × 105 | 9.05 × 10-4 | |
| NS-2# | 1 | 198.8 | 1.427 × 10-4 | 0.8385 | 6.071 × 104 | 8.29 × 10-4 |
| 2 | 196.1 | 5.011 × 10-5 | 0.8915 | 9.385 × 104 | 1.05 × 10-3 | |
| 4 | 183.6 | 3.830 × 10-5 | 0.9385 | 3.809 × 105 | 9.87 × 10-3 | |
| 7 | 171.4 | 3.783 × 10-4 | 0.9425 | 5.215 × 105 | 1.01 × 10-3 | |
| 14 | 148.4 | 4.342 × 10-3 | 0.9478 | 4.932 × 105 | 9.08 × 10-4 | |
| NS-3# | 1 | 193.4 | 8.350 × 10-5 | 0.8461 | 1.814 × 104 | 6.85 × 10-4 |
| 2 | 190.1 | 4.959 × 10-5 | 0.9182 | 1.428 × 105 | 1.15 × 10-3 | |
| 4 | 179.4 | 4.756 × 10-5 | 0.9369 | 1.956 × 105 | 9.09 × 10-4 | |
| 7 | 166.5 | 5.263 × 10-5 | 0.9413 | 2.121 × 105 | 5.82 × 10-4 | |
| 14 | 143.9 | 7.236 × 10-5 | 0.9443 | 1.481 × 105 | 6.28 × 10-4 | |
图7
图7
不同Cu含量管线钢在灭菌和接菌NS4溶液中
Fig.7
Time dependence of
2.3 生物膜分析
图8为3种实验用钢在接菌NS4溶液中浸泡14 d后,样品表面细菌的活/死染色结果。用于染色实验的荧光染料SYTO-9具有高度亲脂性和细胞膜穿透性,与活细胞中的酯酶反应生成钙黄绿素,发出强烈的绿色荧光;而PI不能穿透活细胞的细胞膜结构,但可以透过死亡细胞的细胞膜变性区到达细胞核,与DNA结合发出红色荧光,因此在CLSM下观察到的活性SRB发出绿色荧光点,失活的SRB呈现红色荧光。可以看出,1#钢表面上有大量死细菌,2#钢表面上有少量死细菌,而3#钢表面上未观察到死细菌的存在,即随着Cu含量的提高,钢的杀菌性能显著提高。
图8
图8
不同Cu含量管线钢在接菌NS4溶液中浸泡14 d后的CLSM像
Fig.8
CLSM images of Cu-bearing steels with different Cu contents immersed in SRB inoculated solution for 14 d
(a) 1# (b) 2# (c) 3#
表3 接菌NS4溶液中的EIS拟合结果
Table 3
| Steel | Time d | Rs Ω·cm2 | Qf | Rf Ω·cm2 | Qdl | Rct Ω·cm2 | |||
|---|---|---|---|---|---|---|---|---|---|
| Yf / S·s n ·cm-2 | nf | Ydl / (S·s n ·cm-2) | ndl | ||||||
| SRB-1# | 1 | 216.4 | 5.25 × 10-3 | 0.9102 | 6.627 × 104 | 4.71 × 10-4 | |||
| 2 | 201.8 | 8.80 × 10-4 | 0.9100 | 5.509 × 104 | 8.09 × 10-4 | ||||
| 4 | 180.2 | 7.73 × 10-3 | 0.4713 | 47.9 | 3.05 × 10-3 | 0.9586 | 3.897 × 104 | 3.79 × 10-4 | |
| 7 | 172.5 | 1.07 × 10-2 | 0.4280 | 46.5 | 4.51 × 10-3 | 0.9582 | 1.451 × 105 | 2.43 × 10-5 | |
| 14 | 153.0 | 7.92 × 10-5 | 0.4683 | 25.5 | 2.38 × 10-3 | 0.9599 | 4.578 × 105 | 9.57 × 10-4 | |
| SRB-2# | 1 | 223.6 | 5.50 × 10-4 | 0.9134 | 5.686 × 104 | 5.74 × 10-4 | |||
| 2 | 205.9 | 6.30 × 10-4 | 0.9107 | 5.136 × 104 | 1.34 × 10-3 | ||||
| 4 | 184.0 | 5.25 × 10-3 | 0.5171 | 62.0 | 6.83 × 10-5 | 0.8613 | 3.695 × 104 | 7.08 × 10-4 | |
| 7 | 166.1 | 9.96 × 10-3 | 0.4871 | 64.3 | 4.91 × 10-3 | 0.9679 | 7.404 × 104 | 1.95 × 10-5 | |
| 14 | 150.5 | 9.37 × 10-2 | 0.4326 | 68.4 | 2.88 × 10-3 | 0.9383 | 3.659 × 105 | 6.33 × 10-4 | |
| SRB-3# | 1 | 336.7 | 1.202 × 10-4 | 0.8096 | 5.097 × 104 | 5.74 × 10-4 | |||
| 2 | 311.7 | 7.821 × 10-5 | 0.8763 | 4.003 × 104 | 1.34 × 10-3 | ||||
| 4 | 184.3 | 5.62 × 10-3 | 0.5385 | 46.8 | 3.358 × 10-3 | 0.9654 | 3.486 × 104 | 3.32 × 10-5 | |
| 7 | 169.9 | 8.93 × 10-3 | 0.4411 | 57.3 | 5.402 × 10-3 | 0.9658 | 4.555 × 104 | 1.35 × 10-5 | |
| 14 | 154.8 | 7.00 × 10-5 | 0.4417 | 63.0 | 4.840 × 10-3 | 0.9599 | 1.068 × 105 | 1.07 × 10-3 | |
2.4 腐蚀产物形貌及成分分析
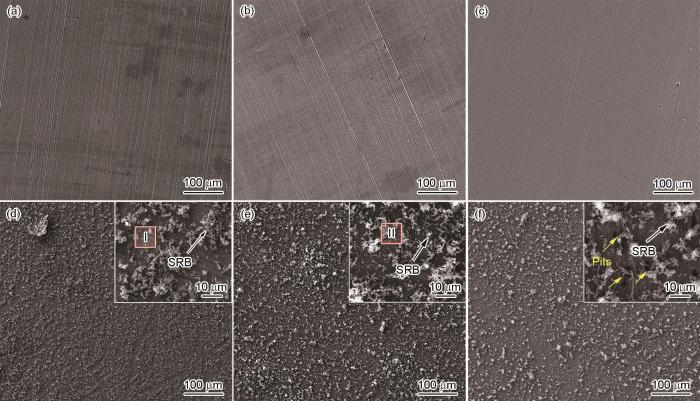
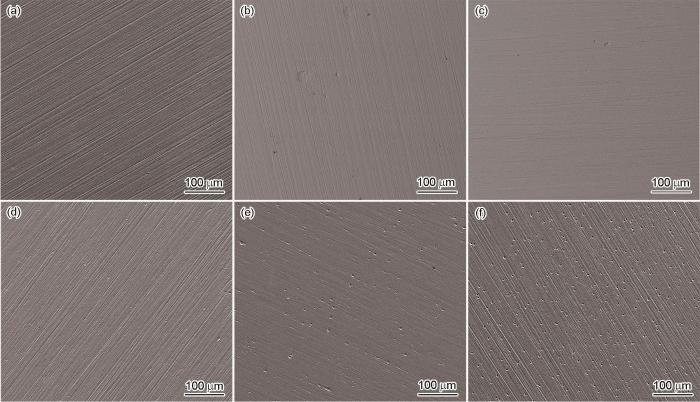
3种实验用钢试样在无菌及有菌介质中浸泡14 d后的表面形貌如图9所示。在无菌溶液中,腐蚀产物较少,表面砂纸打磨的划痕清晰可见,说明3种实验用钢表面只发生了较轻微的均匀腐蚀。样品表面上可观察到极个别的点蚀坑,这可能是由于钢中夹杂物的溶解造成的。在接种SRB的溶液中浸泡14 d后,3种实验用钢的表面上均被腐蚀产物完全覆盖,形成了较厚的腐蚀产物层。在高倍SEM下观察可发现腐蚀产物分为2层:内层较为致密,紧贴于样品表面;外层为松散的、不连续的附着于表面的短杆状SRB及其菌落以及由细菌、腐蚀产物等组成的混合物。另外,2#和3#钢表面上的划痕已被内层腐蚀产物覆盖,而1#钢表面上仍可见砂纸打磨的划痕,也说明1#钢表面生成的腐蚀产物较少;在3#钢表面上还能观察到菌落下方有大量的腐蚀坑(图9f白色箭头),说明3#钢已发生了点蚀。
图9
图9
不同Cu含量管线钢在灭菌和接菌NS4溶液中浸泡14 d后的表面腐蚀产物形貌SEM像
Fig.9
SEM images of Cu-bearing steels with different Cu contents immersed in sterile (a-c) and SRB inoculated (d-f) NS4 solutions for 14 d (Insets are the high magnification morphologies of the corrosion products on the steel surfaces. Red squares in the insets denote the element analysis locations) (a, d) 1# (b, e) 2# (c, f) 3#
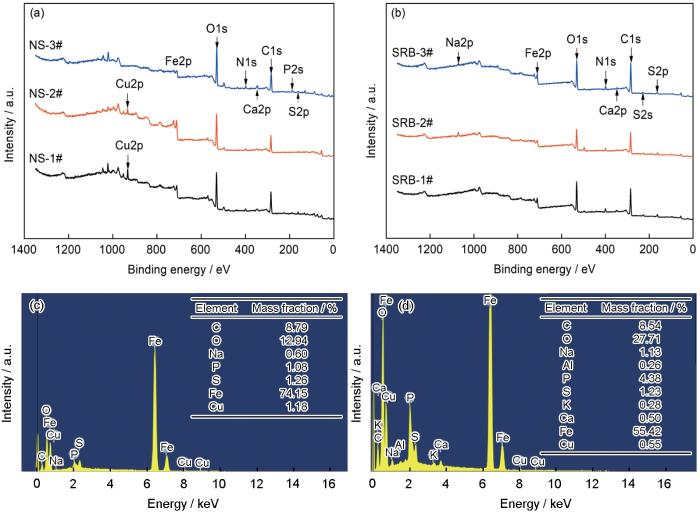
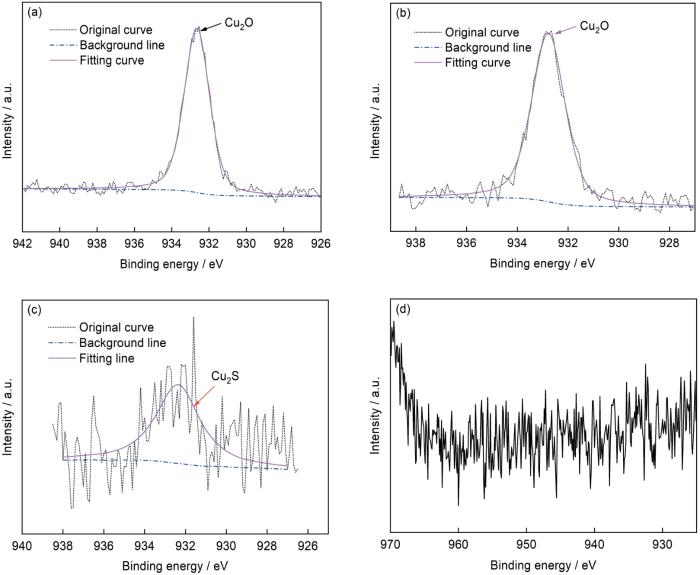
利用XPS对钢表面上的腐蚀产物进行成分分析,结果如图10a和b所示。可以看到,在无菌环境中,3种实验用钢的表面上均探测到C、Fe、O等元素,这主要是钢的厌氧腐蚀产生的Fe的氧化物和氢氧化物;1#和2#钢的腐蚀产物中还有一定量的Cu元素,说明钢中有一定量的Cu溶出。在接种SRB的溶液中,同样探测到了C、Fe、O和少量的无机盐离子。此外,还检测到大量的S元素的存在,它主要来自于SRB的生理活动,在腐蚀产物中以FeS等硫化物的形式存在,这说明SRB参与了腐蚀过程[22,23]。需要指出的是,由于接菌环境中的腐蚀产物较多,采用XPS在1#和2#样品的腐蚀产物中只探测到了少量的Cu,通过SEM-EDS (如图10c和d,对应图9d和e方框区域I、II)可以探测到1#和2#钢表面的腐蚀产物中有Cu的存在。
图10
图10
不同Cu含量管线钢在无菌和接菌NS4溶液中浸泡14 d后表面腐蚀产物的XPS全谱和EDS分析结果
Fig.10
XPS survey spectra of Cu-bearing steels with different Cu contents in sterile (a) and SRB inoculated (b) NS4 solutions for 14 d, and EDS analyses of 1# (c) and 2# (d) steels (boxes I and II in Fig.9) in SRB inoculated solution after 14 d immersion
图11是1#和2#管线钢在无菌和有菌环境中浸泡14 d后表面腐蚀产物中Cu的精细谱。结果表明,在无菌环境中,1#和2#钢表面的腐蚀产物中的Cu主要以Cu2O的形式存在,其对应的峰位为932.7 eV。而在接种SRB的环境中,1#钢表面的Cu的主要存在形式是Cu2S,其对应的峰位为932.4 eV,而2#钢表面上的Cu2p图谱为不可拟合的噪声信号,这可能是由于2#钢中的Cu含量较低导致的。需要注意的是,XPS的探测深度有限,因此该结果只反映了外层腐蚀产物中Cu的化学状态,对于靠近基体的Cu的存在形式仍待进一步研究。
图11
图11
不同Cu含量管线钢在无菌和接菌NS4溶液中浸泡14 d后表面腐蚀产物中Cu的精细谱
Fig.11
High resolution XPS spectra of Cu in the surficial corrosion products on Cu-bearing steels with different Cu contents after immersion in sterile (a, b) and SRB inoculated (c, d) NS4 solutions for 14 d
(a, c) 1# (b, d) 2#
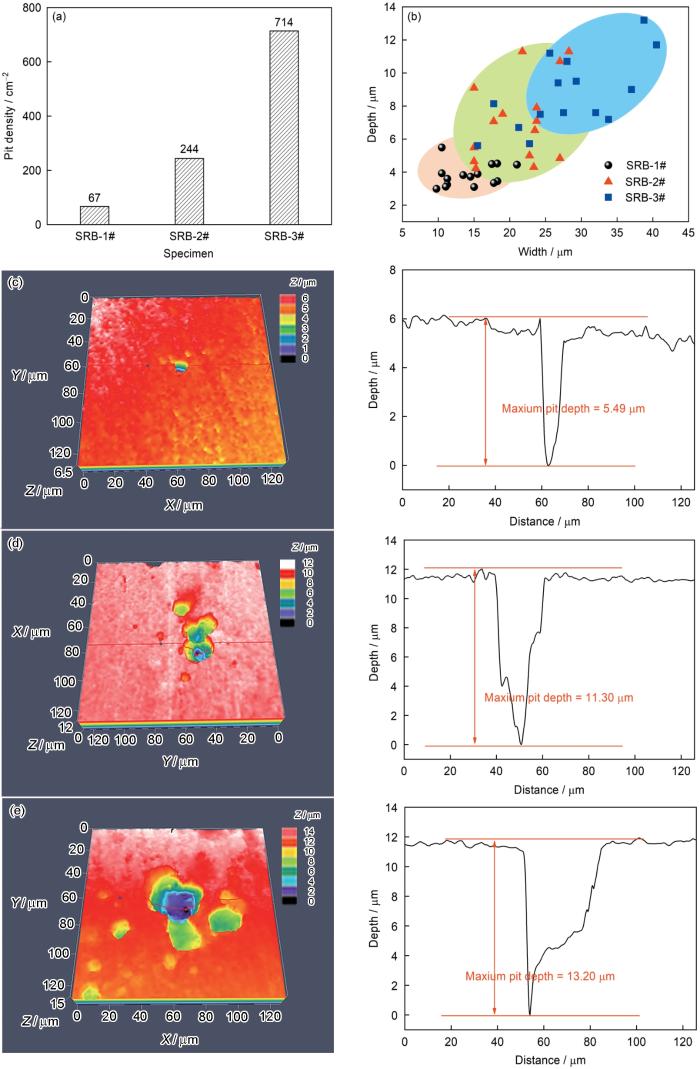
去除腐蚀产物后的样品表面形貌如图12所示。在无菌溶液中,1#和2#钢表面上的砂纸打磨痕迹清晰可见,3#钢表面上的划痕已被腐蚀掉,说明3#钢表面发生了更严重的均匀腐蚀。在接种SRB的环境中浸泡后的结果表明,3种实验用钢均发生了明显的点蚀。然而,随着钢中Cu含量的升高,点蚀程度减轻。利用SEM和CLSM定量分析接菌环境中各样品表面上的点蚀坑密度、直径和深度,结果列于图13。如图13a所示,随着Cu含量的降低,样品表面上的点蚀坑密度急剧升高,1#、2#、3#钢表面上的点蚀坑密度分别是67、244和714 cm-2。随机选取15个点蚀坑,利用CLSM测量其直径和深度,统计结果及其中最大的点蚀坑形貌示于图13b~e。可以看到,Cu含量的升高能显著减小钢的表面上的点蚀坑深度及直径,1#、2#、3#钢表面上的最大点蚀坑深度分别是5.49、11.30和13.20 µm。以上结果说明,当Cu含量达到1.34%时,已基本上能抑制实验用钢发生点蚀。
图12
图12
不同Cu含量管线钢在无菌和接菌NS4溶液中浸泡14 d后去除腐蚀产物后的表面形貌
Fig.12
Surface morphologies with removal of corrosion products on Cu-bearing steels with different Cu contents after immersion in sterile (a-c) and SRB inoculated (d-f) NS4 solutions for 14 d (a, d) 1# (b, e) 2# (c, f) 3#
图13
图13
不同Cu含量管线钢在接菌NS4溶液中浸泡14 d后的表面点蚀坑统计结果
Fig.13
Pits statistics on Cu-bearing steels with different Cu contents after immersion in SRB inoculated NS4 solution for 14 d
(a) pit density (b) pit depth
(c-e) pit diameters and the largest pits on 1# (c), 2# (d), and 3# (e) steels, respectively
3 分析与讨论
上述研究表明,不论是在无菌还是有菌环境中,Cu合金化管线钢的耐蚀性能均有所提高。在无菌环境中,3种实验用钢主要发生了缓慢的均匀腐蚀,且随着Cu含量的升高,钢的耐蚀性能也随之提高。XPS结果表明,Cu在腐蚀产物中以Cu2O的形式存在。根据文献[24~26]报道,含Cu钢在腐蚀过程中,基体中的Cu可以通过溶解和再沉积在材料表面上积累形成Cu、Cu2O等富Cu产物层,该富集层的存在不但可以作为阻挡层阻碍腐蚀过程中的物质交换,从而抑制钢的电化学反应,还能作为保护性腐蚀产物(如Fe3O4等)的表面吸附位点,进一步降低钢的腐蚀速率。从
在接种SRB的环境中,SRB显著促进了钢的腐蚀,特别是点蚀(图3、9和10)。与无菌环境不同的是,SRB的代谢会产生大量还原态的S,这些还原态S可以与钢基体溶解出的Fe2+等阳离子结合,形成特征腐蚀产物FeS等铁硫化物[27] (图9和10),这些铁硫化物与SRB细胞以及由SRB代谢产生的胞外聚合物(EPS)共同构成覆盖在样品表面的腐蚀产物膜。如图9所示,腐蚀产物膜并不稳定,其间存在大量孔隙和微裂纹,容易破裂;同时腐蚀产物层的导电性也由于具有半导体特性的FeS等的存在而升高,从而在电解质与Fe基体之间形成连续的电子传递路径,使得代谢产物如FeS (阴极)和由于产物膜破裂而裸露的钢基体(阳极)之间形成了一个电偶对,促进了钢的局部腐蚀,这也是有菌环境中腐蚀以点蚀为主的重要原因之一[28,29]。本工作中,如图12和13,随着Cu含量的提高,钢试样表面的点蚀坑密度和深度均有明显下降。Baba等[30]认为,含Cu钢在含有H2S的水溶液腐蚀过程中,可以在钢的表面形成致密的复杂硫化物,如(Fe, Cu)S,它可以作为抑制钢进一步腐蚀的屏障;此外,Cu在FeS薄膜中的富集还会改变FeS的半导体性能[31],导致其导电性能下降,进而降低电解质和Fe基体之间的电子传输效率,抑制含Cu钢的腐蚀。
式中,
Cu及其合金化金属的优异抗菌性能已经得到了广泛的验证[35~37]。一般认为,其在腐蚀过程中溶出的Cu2+和Cu+是其具有抗菌性能的重要原因[38~40]。研究[41,42]表明,Cu2+和Cu+可以破坏细菌细胞膜的完整性,穿透细胞膜使蛋白凝固,造成细胞内酶的失活,同时还会参与催化Fenton反应,产生活性氧(reactive oxygen species,ROS),进而导致SRB细菌凋亡。值得注意的是,也有研究[43,44]认为,在SRB腐蚀环境中,钢的基体释放出的铜离子会与SRB代谢产生的H2S反应生成难溶的Cu2S和CuS,从而影响了铜离子的杀菌效果。但是在本工作中,虽然在腐蚀产物中同样含有Cu2S,但是含Cu钢(1#、2#)仍然具有相当强的杀菌性能,这可能是由于碳钢较不锈钢更易腐蚀,因此就可能从钢中析出更多铜离子,从而保证了材料的杀菌性能。并且,当Cu含量达到1.34%时,其表层腐蚀产物中也能探测到少量Cu的存在,而2#钢探测不到,说明1#管线钢有更多的铜离子溶出,因而其抗菌性能更好。尽管如此,Cu合金化管线钢的杀菌机制仍需要进一步研究。
综上,含Cu管线钢的腐蚀过程可简述如下:在无菌环境中,钢在腐蚀过程中,基体中的Cu会不断地溶解形成Cu2O沉积在钢的表面形成保护性腐蚀产物层,抑制了钢的继续腐蚀;在有菌环境中,Cu不断从基体溶解释放,除了可以改善腐蚀产物层的成分结构,降低腐蚀产物和基体之间的电偶腐蚀作用,还可以使附着在钢表面上的细菌失活,且随着Cu含量的升高,杀菌效果越强,从而抑制了细菌从钢的基体直接获取电子,减轻了钢的微生物腐蚀。
4 结论
(1) 不同Cu含量管线钢(0、0.70%、1.34%)在无菌和接种SRB的NS4溶液中浸泡14 d后,呈现不同的腐蚀形貌。无菌环境中,3种管线钢基体均未见明显腐蚀;硫酸盐还原菌促进了管线钢的腐蚀,腐蚀形式主要为点蚀。
(2) Cu的添加提高了管线钢在无菌和接种SRB环境中的耐蚀性能,且Cu含量越高,耐蚀性能越强。当Cu含量从0增加到0.70%时,试样表面的点蚀坑密度从714 cm-2下降到244 cm-2,而最大点蚀深度分别是13.20和11.30 µm;当Cu含量提高到1.34%时,点蚀坑密度进一步下降到67 cm-2,最大点蚀坑深度则下降到5.49 µm。
(3) 含Cu管线钢在腐蚀过程中持续溶出的铜离子在接种SRB环境中的杀菌作用和其对腐蚀产物层的改善作用,以及其在无菌环境中所形成的Cu2O等保护性腐蚀产物是钢具有更佳耐蚀性能的主要原因。
参考文献
Microbiologically influenced corrosion of underground pipelines under the disbonded coatings
[J].
Effect of biofilm on cast iron pipe corrosion in drinking water distribution system: Corrosion scales characterization and microbial community structure investigation
[J].
Corrosion of copper and steel alloys in a simulated underground storage-tank sump environment containing acid-producing bacteria
[J].
Ship ballast tanks a review from microbial corrosion and electrochemical point of view
[J].
Bibliometric analysis of microbiologically influenced corrosion (MIC) of oil and gas engineering systems
[J].Managing microbiologically influenced corrosion (MIC) is both an economic and technological challenge for the oil and gas industry. There are studies and data generated regarding the corrosion mechanism, microbial species involved, and chemicals that may enhance/inhibit MIC. However, these data are diffuse, sometimes having contradictory conclusions and ignoring one or more key factors that drive MIC. This paper investigates the evolution of MIC knowledge in the past decades by conducting a bibliometric analysis of the literature. The paper also identifies current knowledge gaps and proposes future research directions. Although MIC mechanisms, monitoring, and control have been active areas of research in recent years, linking microbiological activities, the chemical environment (e.g., produced water lines vs. crude lines), and the corrosion mechanisms is still an important knowledge gap. The importance of a coordinated multidisciplinary approach to develop integrated knowledge, MIC mechanistic models, and integration of these factors in effective decision-making is also discussed in this paper.
Methane emissions from the 2015 Aliso Canyon blowout in Los Angeles, CA
[J].Single-point failures of natural gas infrastructure can hamper methane emission control strategies designed to mitigate climate change. The 23 October 2015 blowout of a well connected to the Aliso Canyon underground storage facility in California resulted in a massive release of natural gas. Analysis of methane and ethane data from dozens of plume transects, collected during 13 research-aircraft flights between 7 November 2015 and 13 February 2016, shows atmospheric leak rates of up to 60 metric tons of methane and 4.5 metric tons of ethane per hour. At its peak, this blowout effectively doubled the methane emission rate of the entire Los Angeles basin and, in total, released 97,100 metric tons of methane to the atmosphere. Copyright © 2016, American Association for the Advancement of Science.
Corrosion at Prudhoe Bay—A lesson on the line
[J].
Critical review: Microbially influenced corrosion of buried carbon steel pipes
[J].
Effects of biogenic H2S on the microbiologically influenced corrosion of C1018 carbon steel by sulfate reducing Desulfovibrio vulgaris biofilm
[J].
A review: microbiologically influenced corrosion and the effect of cathodic polarization on typical bacteria
[J].
Microbiologically influenced corrosion and current mitigation strategies: A state of the art review
[J].
Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria
[J].The bioelectric effect, in which electric fields are used to enhance the efficacy of biocides and antibiotics in killing biofilm bacteria, has been shown to reduce the very high concentrations of these antibacterial agents needed to kill biofilm bacteria to levels very close to those needed to kill planktonic (floating) bacteria of the same species. In this report, we show that biofilm bacteria are readily killed by an antibiotic on all areas of the active electrodes and on the surfaces of conductive elements that lie within the electric field but do not themselves function as electrodes. Considerations of electrode geometry indicate that very low (< 100 microA/cm2) current densities may be effective in this electrical enhancement of antibiotic efficacy against biofilm bacteria, and flow experiments indicate that this bioelectric effect does not appear to depend entirely on the possible local electrochemical generation of antibacterial molecules or ions. These data are expected to facilitate the use of the bioelectric effect in the prevention and treatment of device-related bacterial infections that are caused by bacteria that grow in biofilms and thereby frustrate antibiotic chemotherapy.
Effect of copper addition in carbon steel on biocorrosion by sulfate-reducing bacteria in solution
[J].
Study on microbiologically influenced corrosion behavior of novel Cu-bearing pipeline steels
[J].
新型含Cu管线钢的微生物腐蚀行为研究
[J].
Microbial corrosion resistance of a novel Cu-bearing pipeline steel
[J].Microbiologically influenced corrosion (MIC) is becoming a serious problem for buried pipelines. Developing environmentally friendly strategies for MIC control is increasingly urgent in oil/gas pipeline industry. Copper (Cu) in steels can not only provide aging precipitation strengthening, but also kill bacterium, offering a special biofunction to steels. Based on the chemical composition of traditional X80 pipeline steel, two Cu-bearing pipeline steels (1% Cu and 2% Cu) were fabricated in this study. The microstructure, mechanical properties and antibacterial property against sulphate-reducing bacteria (SRB) and Pseudomonas aeruginosa (P. aeruginosa) were studied. It was found that the novel pipeline steel alloyed by 1%Cu exhibited acicular ferrite microstructure with nano-sized Cu-rich precipitates distribution in the matrix, resulting in better mechanical properties than the traditional X80 steel, and showed good MIC resistance as well. The pitting corrosion resistance of 1% Cu steel in as-aged condition was significantly better than that of X80 steel. A possible antibacterial mechanism of the Cu-bearing pipeline steel was proposed.
Suppression of surface hot shortness due to Cu in recycled steels
[J].
Microbiologically induced corrosion of X80 pipeline steel in an acid soil solution: (I) Electrochemical analysis
[J].
酸性土壤浸出液中X80钢微生物腐蚀研究: (I)电化学分析
[J].利用微生物和电化学方法研究了X80管线钢在一种酸性土壤浸出液中的硫酸盐还原菌 (SRB) 腐蚀电化学特征。结果表明,刚接种到酸性土壤浸出液中的SRB需要重新适应环境,该过程导致细菌数量大幅降低;接菌土壤浸出液中管线钢的开路电位低于灭菌土壤浸出液中的;实验前期活性生物膜对管线钢腐蚀起抑制作用,后期微生物代谢产物促进管线钢的腐蚀;SRB活动改变了金属/溶液的电介质性质,是实验后期促进管线钢腐蚀的重要原因。
The relationship between the impedance of corroding electrode and its polarization resistance determined by a linear voltage sweep technique
[J].
Sulfate reducing bacteria corrosion of pipeline steel in Fe-rich red soil
[J].
富Fe红壤中管线钢的硫酸盐还原菌腐蚀行为
[J].
Variations of microenvironments with and without SRB for steel Q 235 under a simulated disbonded coating
[J].
Surface chemistry and corrosion behaviour of 304 stainless steel in simulated seawater containing inorganic sulphide and sulphate-reducing bacteria
[J].
Corrosion behaviour of copper containing low alloy steels in sulphuric acid
[J].
Accumulation of copper layer on a surface in the anodic polarization of stainless steel containing Cu at different temperatures
[J].
An electrochemical study on the effect of pitting inhibition in weakly alkaline solution by copper addition in pure iron
[J].
Microbiologically induced corrosion of X80 pipeline steel in an acid soil solution: (II) Corrosion morphology and corrosion product analysis
[J].
酸性土壤浸出液中X80钢微生物腐蚀研究: (II) 腐蚀形貌和产物分析
[J].利用X射线光电子能谱分析 (XPS) 和扫描电子显微镜 (SEM) 研究了X80管线钢在一种酸性土壤浸出液中硫酸盐还原菌 (SRB) 腐蚀的产物成分和形貌。结果表明,SRB没有改变样品表面腐蚀产物膜的结构,但其生理过程促进了S从氧化态向还原态的转变,并促进了S在腐蚀产物中的沉积;同时代谢产物磷化物也沉积在腐蚀产物中,改变了腐蚀产物的成分。SRB提高了管线钢局部腐蚀的敏感性,使得腐蚀形式从非均匀腐蚀向局部腐蚀转变。SRB代谢产物和细菌/金属间的直接电子转移可能是促进局部腐蚀萌生的主要原因。
Heterogeneous corrosion of mild steel under SRB-biofilm characterised by electrochemical mapping technique
[J].
Corrosion of iron by sulfate-reducing bacteria: New views of an old problem
[J].\n About a century ago, researchers first recognized a connection between the activity of environmental microorganisms and cases of anaerobic iron corrosion. Since then, such microbially influenced corrosion (MIC) has gained prominence and its technical and economic implications are now widely recognized. Under anoxic conditions (e.g., in oil and gas pipelines), sulfate-reducing bacteria (SRB) are commonly considered the main culprits of MIC. This perception largely stems from three recurrent observations. First, anoxic sulfate-rich environments (e.g., anoxic seawater) are particularly corrosive. Second, SRB and their characteristic corrosion product iron sulfide are ubiquitously associated with anaerobic corrosion damage, and third, no other physiological group produces comparably severe corrosion damage in laboratory-grown pure cultures. However, there remain many open questions as to the underlying mechanisms and their relative contributions to corrosion. On the one hand, SRB damage iron constructions indirectly through a corrosive chemical agent, hydrogen sulfide, formed by the organisms as a dissimilatory product from sulfate reduction with organic compounds or hydrogen (“chemical microbially influenced corrosion”; CMIC). On the other hand, certain SRB can also attack iron via withdrawal of electrons (“electrical microbially influenced corrosion”; EMIC),\n viz\n., directly by metabolic coupling. Corrosion of iron by SRB is typically associated with the formation of iron sulfides (FeS) which, paradoxically, may reduce corrosion in some cases while they increase it in others. This brief review traces the historical twists in the perception of SRB-induced corrosion, considering the presently most plausible explanations as well as possible early misconceptions in the understanding of severe corrosion in anoxic, sulfate-rich environments.\n
Effect of Cu addition in pipeline steels on prevention of hydrogen permeation in mildly sour environments
[J].
Effect of copper on the protectiveness of iron sulfide films
[J].
Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria
[J].
Carbon source starvation triggered more aggressive corrosion against carbon steel by the Desulfovibrio vulgaris biofilm
[J].
Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria
[J].Sulfate reducing bacteria (SRB) are often the culprits of microbiologically influenced corrosion (MIC) in anoxic environments because sulfate is a ubiquitous oxidant. MIC of carbon steel caused by SRB is the most intensively investigated topic in MIC because of its practical importance. It is also because biogenic sulfides complicate mechanistic SRB MIC studies, making SRB MIC of carbon steel is a long-lasting topic that has generated considerable confusions. It is expedient to think that biogenic H2S secreted by SRB acidifies the broth because it is an acid gas. However, this is not true because endogenous H2S gets its H+ from organic carbon oxidation and the fluid itself in the first place rather than an external source. Many people believe that biogenic H2S is responsible for SRB MIC of carbon steel. However, in recent years, well designed mechanistic studies provided evidence that contradicts this misconception. Experimental data have shown that cathodic electron harvest by an SRB biofilm from elemental iron via extracellular electron transfer (EET) for energy production by SRB is the primary cause. It has been demonstrated that when a mature SRB biofilm is subjected to carbon source starvation, it switches to elemental iron as an electron source and becomes more corrosive. It is anticipated that manipulations of EET related genes will provide genetic-level evidence to support the biocathode theory in the future. This kind of new advances will likely lead to new gene probes or transcriptomics tools for detecting corrosive SRB strains that possess high EET capabilities.
Antibacterial mechanism of copper-bearing antibacterial stainless steel against E. coli
[J].
Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: In vitro and in vivo evaluations
[J].
Metallic copper as an antimicrobial surface
[J].Bacteria, yeasts, and viruses are rapidly killed on metallic copper surfaces, and the term “contact killing” has been coined for this process. While the phenomenon was already known in ancient times, it is currently receiving renewed attention. This is due to the potential use of copper as an antibacterial material in health care settings. Contact killing was observed to take place at a rate of at least 7 to 8 logs per hour, and no live microorganisms were generally recovered from copper surfaces after prolonged incubation. The antimicrobial activity of copper and copper alloys is now well established, and copper has recently been registered at the U.S. Environmental Protection Agency as the first solid antimicrobial material. In several clinical studies, copper has been evaluated for use on touch surfaces, such as door handles, bathroom fixtures, or bed rails, in attempts to curb nosocomial infections. In connection to these new applications of copper, it is important to understand the mechanism of contact killing since it may bear on central issues, such as the possibility of the emergence and spread of resistant organisms, cleaning procedures, and questions of material and object engineering. Recent work has shed light on mechanistic aspects of contact killing. These findings will be reviewed here and juxtaposed with the toxicity mechanisms of ionic copper. The merit of copper as a hygienic material in hospitals and related settings will also be discussed.
Study on behaviour and mechanism of Cu2+ ion release from Cu bearing antibacterial stainless steel
[J].
Relationship between surface properties and antibacterial behavior of wire arc spray copper coatings
[J].
Laboratory investigation of the microbiologically influenced corrosion (MIC) resistance of a novel Cu-bearing 2205 duplex stainless steel in the presence of an aerobic marine Pseudomonas aeruginosa biofilm
[J].
Inhibition of Staphylococcus aureus biofilm by a copper-bearing 317L-Cu stainless steel and its corrosion resistance
[J].
Mechanism of copper surface toxicity in vancomycin-resistant enterococci following wet or dry surface contact
[J].\n Contaminated touch surfaces have been implicated in the spread of hospital-acquired infections, and the use of biocidal surfaces could help to reduce this cross-contamination. In a previous study we reported the death of aqueous inocula of pathogenic\n Enterococcus faecalis\n or\n Enterococcus faecium\n isolates, simulating fomite surface contamination, in 1 h on copper alloys, compared to survival for months on stainless steel. In our current study we observed an even faster kill of over a 6-log reduction of viable enterococci in less than 10 min on copper alloys with a “dry” inoculum equivalent to touch contamination. We investigated the effect of copper(I) and copper(II) chelation and the quenching of reactive oxygen species on cell viability assessed by culture and their effects on genomic DNA, membrane potential, and respiration\n in situ\n on metal surfaces. We propose that copper surface toxicity for enterococci involves the direct or indirect action of released copper ionic species and the generation of superoxide, resulting in arrested respiration and DNA breakdown as the first stages of cell death. The generation of hydroxyl radicals by the Fenton reaction does not appear to be the dominant instrument of DNA damage. The bacterial membrane potential is unaffected in the early stages of wet and dry surface contact, suggesting that the membrane is not compromised until after cell death. These results also highlight the importance of correct surface cleaning protocols to perpetuate copper ion release and prevent the chelation of ions by contaminants, which could reduce the efficacy of the surface.\n
Corrosion of antibacterial Cu-bearing 316L stainless steels in the presence of sulfate reducing bacteria
[J].