具备低密度以及生物可降解性的Mg及镁合金在结构材料的轻量化和医用植入材料的自降解上具有广阔的发展前景[1]。然而,Mg及其合金因其很负的电极电位和高的化学活性而极易发生腐蚀,这在很大程度上限制了其应用。此外,第二相和杂质的存在也会加速镁合金的腐蚀;在模拟体液或者机体内环境中,镁合金特别容易吸附溶液中的Cl- [2~4]产生可溶性氯化物而被腐蚀。镁合金过快的降解速率导致镁合金在服役期内过早失去机械强度而提前失效,无法满足或匹配骨组织愈合所需的时间。同时,镁合金在腐蚀过程中通常会产生大量H2,并使其局部碱化[5],进而影响植入体周围细胞的生长,甚至可能会引起碱中毒现象。因此,将缓蚀剂与化学转化膜相结合,通过其与金属离子的吸附性或络合性对转化膜进行诱导,进而控制和降低镁合金的降解速率是非常有必要的。
张军等[21]采用静态失重和分子模拟相结合的方法研究了半胱氨酸、缬氨酸和丙氨酸3种氨基酸的缓蚀效果,结果表明:随着缓蚀剂分子反应活性的增加,缓蚀剂分子在金属表面的吸附强度增加,缓蚀效果增强,3种缓蚀剂的缓蚀效率顺序为半胱氨酸>缬氨酸>丙氨酸。El-Hafez等[22]通过电化学阻抗法及极化曲线法研究了半胱氨酸、N-乙酰半胱氨酸和甲硫氨酸在NaCl溶液中对Cu-Ni合金的腐蚀抑制情况,认为氨基酸的腐蚀抑制作用是由于其在合金表面的吸附。Zhao等[23]在0.5 mol/L NaCl溶液中研究了含硫氨基酸(L-半胱氨酸(CYS)和L-蛋氨酸(MET))作为环境友好型自组装膜(SAMs)对316L钢的缓蚀效果,结果表明,CYS和MET分子的自组装膜可以作为混合型缓蚀剂,保护316L钢在NaCl溶液中免受侵蚀性离子的侵蚀,且随着氨基酸(CYS或MET)浓度的增加,抑制作用不断增强。
Wang等[24]提出了氨基酸对抑制纯Mg在磷酸盐缓冲溶液中的降解有重要作用,发现氨基酸分子与Mg2+和HPO
本工作通过氨基酸诱导镁合金表面Ca-P涂层,研究氨基酸极性官能团氨基、羧基的作用和苯丙氨酸中苯环、甲硫氨酸中硫键、天冬酰胺中的酰胺键的吸附效果。
1 实验方法
1.1 涂层制备
挤压态AZ31镁合金板材的主要化学成分(质量分数,%)为:Al 3.19,Zn 0.81,Mn 0.334,Si 0.02,Fe 0.005,Cu 0.05,Ca 0.04,Be 0.1,Mg余量。样品切割为20 mm × 20 mm × 5 mm的矩形平行六面体。样品用SiC水磨砂纸打磨到2500号磨至表面无明显划痕。先使用蒸馏水冲洗样品表面杂质、再用无水乙醇冲洗样品表面油污,并采用热风干燥后真空保存。
选定下列3种氨基酸:苯丙氨酸(Phenylalanine,Phe)、甲硫氨酸(Methionine,Met)和天冬酰胺(Asparagine,Asn)。
Ca-P涂层制备:将0.25 mol/L的CaCl2和KH2PO4通过超声搅拌溶解在去离子水中,随后,将打磨好的样品浸入上述混合溶液中在60℃恒温水浴搅拌处理30 min,取出样品后,用蒸馏水冲洗,并用热风干燥,最终得到Ca-P涂层。
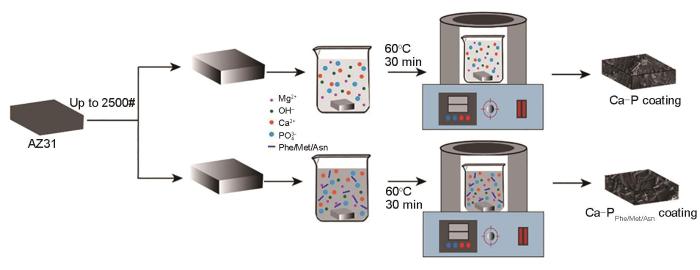
氨基酸Ca-P涂层制备:将0.25 mol/L的CaCl2和KH2PO4通过超声搅拌溶解在去离子水中,并分别添加12 mmol/L的苯丙氨酸/甲硫氨酸/天冬酰胺,随后,将打磨好的样品浸入混合溶液中在60℃恒温水浴搅拌处理30 min,取出样品后用蒸馏水冲洗,并用热风干燥,最终得到3种氨基酸Ca-P涂层:苯丙氨酸Ca-P (Ca-PPhe)涂层、甲硫氨酸Ca-P (Ca-PMet)涂层和天冬酰胺Ca-P (Ca-PAsn)涂层。其制备流程见图1。
图1
图1
AZ31镁合金表面Ca-P涂层和3种氨基酸(苯丙氨酸、甲硫氨酸和天冬酰胺)诱导Ca-P涂层(Ca-PPhe、Ca-PMet和Ca-PAsn)制备流程图
Fig.1
Preparation diagram of Ca-P coating and its amino acids (Phenylalanine (Phe), Methionine (Met), and Asparagine (Asn)) modified coatings (Ca-PPhe, Ca-PMet,and Ca-PAsn, respectively) on AZ31 Mg alloy
1.2 腐蚀表征
使用PAR Model 2273电化学工作站获得样品的电化学阻抗谱(EIS)和动电位极化(PDP)曲线,以评估三电极装置(以暴露面积为1 cm2的样品为工作电极,饱和甘汞(SCE)电极为参比电极,Pt电极为对电极)对样品的腐蚀行为。实验在室温下Hank's溶液中进行。首先,在600 s内建立了稳定的开路电位;在扰动电位为10 mVSCE、频率范围为100 kHz~0.01 Hz、浸泡时间为600 s的短延迟后进行EIS分析,EIS数据用ZSimpWin软件拟合;以2 mV/s的扫描速率记录极化曲线,用Versatudio 和Origin Pro 9.1对电化学参数(腐蚀电位(Ecorr)、腐蚀电流密度(icorr)和Tafel斜率(βa、βc))进行拟合。Rp使用Stern-Geary方程计算:
析氢速率的测量方法:将样品置于500 mL装有Hank's溶液的烧杯中,通过倒置的漏斗连接酸式滴定管,整个实验在(37.0 ± 0.5)℃的水浴锅中进行,并通过排水集气法在表面完全暴露的情况下间歇地测量滴定管中的水位。通过单位时间单位面积释放H2的体积来反映腐蚀反应的速率及进程。
1.3 表面分析与表征
利用Nova Nano SEM 450扫描电子显微镜(SEM)及其附带的能谱仪(EDS)观察和分析样品的微观表面形貌和元素组成。通过扫描样品的横截面,确定涂层的厚度和元素分布。在SEM测试之前,样品表面经过真空镀金的导电处理。
采用Rigaku D/MAX-2500 X射线衍射仪(XRD)对涂层进行结构分析,采用CuKα靶(波长λ = 0.154 nm),其扫描范围为5°~80°,扫描速率为8°/min。通过Nicolet 380 Fourier红外光谱(FTIR)表征涂层的官能团以及化学键,实验参数为:背景材料KBr,测量范围4000~400 cm-1。
使用ESCALAB250Xi X射线光电子能谱(XPS)分析涂层所含的元素,进而研究氨基酸的作用。主要实验参数:X射线源为单色化Al靶,样品分析区域700 μm × 300 μm,信息采样深度无机材料< 5 nm。X射线工作功率为150 W,实验数据需要以C为校准峰进行荷电校正,C单质的标准峰为284.8 eV。校正后的数据用XPS PEAK 4.1软件进行分峰拟合。
2 实验结果与讨论
2.1 涂层表面
图2
图2
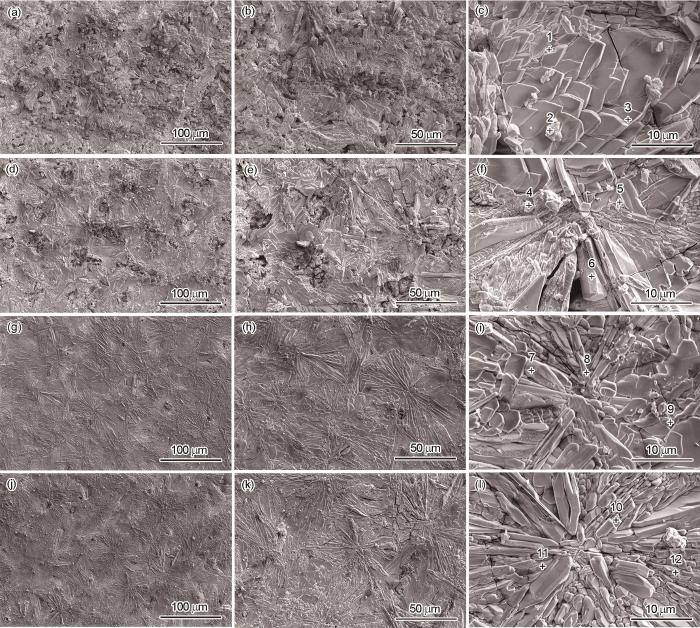
AZ31镁合金表面Ca-P涂层和3种氨基酸诱导Ca-P涂层的SEM像
Fig.2
SEM images of Ca-P coating (a-c), Ca-PPhe coating (d-f), Ca-PMet coating (g-i), and Ca-PAsn coating (j-l) on AZ31 magnesium alloy surface
4种涂层表面的元素组成情况如表1所示。4种涂层表面均检测到Ca、P、C、O和Mg元素。同时,N元素的存在表明氨基酸参与了Ca-P涂层的形成过程。此外,Ca-P、Ca-PPhe、Ca-PMet和Ca-PAsn涂层的Ca和P的含量是递增的。
表1 图2中点1~12的EDS分析结果 (atomic fraction / %)
Table 1
| Point | C | N | O | Mg | Ca | P |
|---|---|---|---|---|---|---|
| 1 | 4.02 | 1.48 | 38.71 | 32.71 | 14.40 | 8.68 |
| 2 | 3.94 | 0.81 | 48.05 | 28.83 | 11.02 | 7.35 |
| 3 | 4.32 | 0.74 | 63.35 | 12.32 | 10.77 | 8.50 |
| 4 | 3.57 | 0.83 | 60.56 | 9.79 | 14.26 | 11.00 |
| 5 | 3.30 | 0.48 | 60.42 | 11.17 | 14.28 | 10.35 |
| 6 | 3.18 | 1.23 | 63.74 | 5.43 | 13.96 | 12.45 |
| 7 | 2.84 | 1.50 | 58.95 | 1.52 | 18.04 | 17.15 |
| 8 | 3.35 | 1.52 | 44.31 | 2.99 | 23.73 | 24.00 |
| 9 | 3.11 | 1.06 | 61.40 | 1.68 | 16.68 | 16.07 |
| 10 | 3.26 | 1.86 | 38.99 | 3.39 | 24.87 | 27.63 |
| 11 | 3.06 | 1.38 | 52.89 | 0.06 | 20.62 | 21.98 |
| 12 | 4.09 | 2.21 | 39.93 | 6.01 | 23.74 | 24.02 |
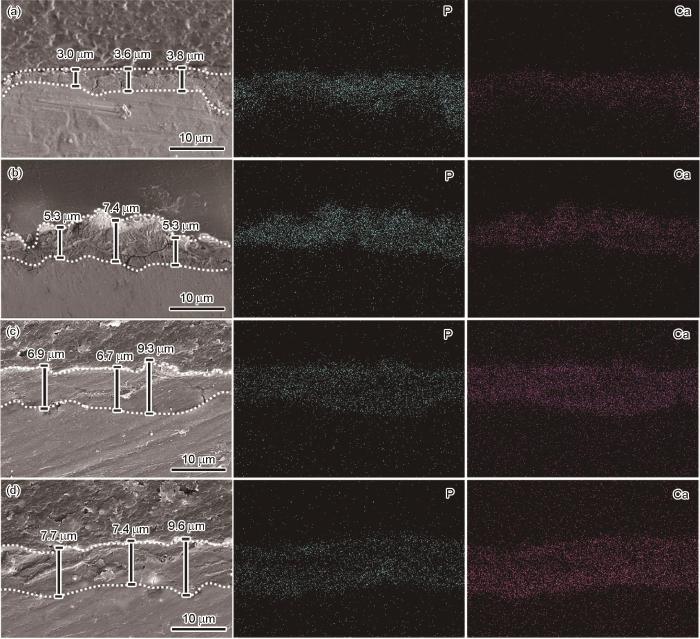
AZ31镁合金表面Ca-P涂层和氨基酸诱导Ca-P涂层的截面图和元素分布如图3所示。Ca-P、Ca-PPhe、Ca-PMet和Ca-PAsn涂层的厚度分别约为(3.47 ± 0.47)、(6.06 ± 0.77)、(7.63 ± 1.70)和(8.23 ± 1.37) μm。结果表明,相较于Ca-P涂层及其他氨基酸诱导的Ca-P涂层,天冬酰胺明显地增加了Ca-P涂层的厚度和致密性。
图 3
图 3
AZ31镁合金表面Ca-P 涂层和3种氨基酸诱导Ca-P涂层截面形貌的SEM像和元素分布
Fig.3
Cross-sectional SEM images and corresponding Ca and P mapping images of Ca-P coating (a), Ca-PPhe coating (b), Ca-PMet coating (c), and Ca-PAsn coating (d) on AZ31 magnesium alloy surface
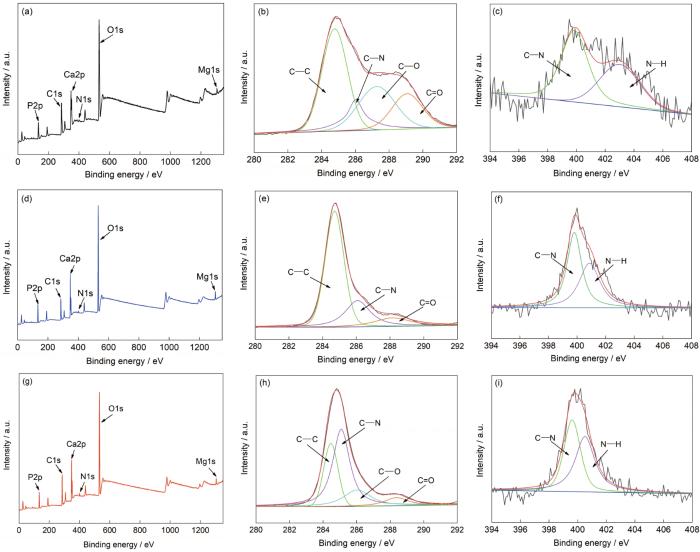
为了了解氨基酸是否参与Ca-P涂层的形成,对3种氨基酸诱导的Ca-P涂层进行了XPS测试。图4a、d和g显示了Ca-PPhe/Met/Asn涂层的XPS测量宽谱。可以看出,3种涂层所含元素并无差异。C、N、O、Mg、Ca和P元素存在于Ca-PPhe/Met/Asn涂层表面上,表明氨基酸参与了Ca-P涂层成膜反应。根据C1s光谱(图4b、e和h),Ca-PPhe/Met/Asn涂层中的C元素均来自氨基酸,其中结合能分别在284.8、285.8、287.6和288.8 eV,分别对应于氨基酸中的C—C键、C—N键、C—O键和C=O键[25]。根据N1s光谱(图4c、f和i),N元素也均来自于氨基酸,其中C—N键和N—H键的结合能分别是399.7和400.4 eV。因此,可以证明氨基酸参与了Ca-P涂层的成膜反应。
图4
图4
3种氨基酸诱导Ca-P涂层的XPS谱宽谱和精细谱
(b, e, h) C1s (c, f, i) N1s
Fig.4
XPS survey plots (a, d, g) and high-resolution spectra (b, c, e, f, h, i) of Ca-PPhe coating (a-c), Ca-PMet coating (d-f), and Ca-PAsn coating (g-i)
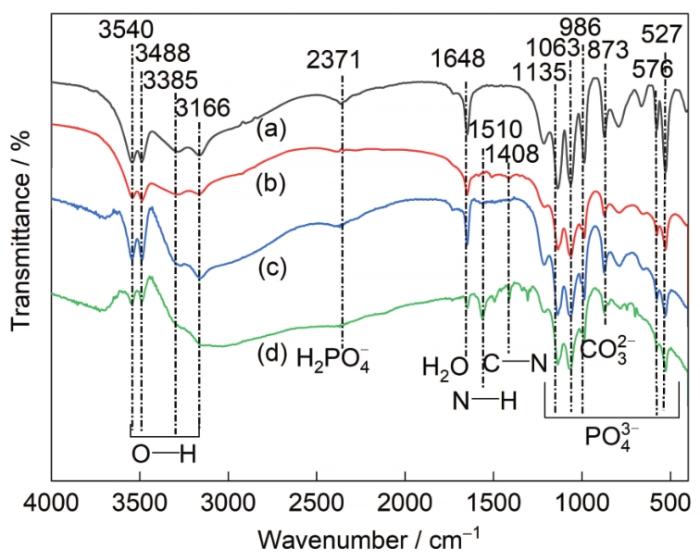
图5为AZ31镁合金表面Ca-P涂层和3种氨基酸诱导Ca-P涂层的FTIR。如图所示,4种样品的FTIR曲线在峰的数目和位置上没有明显的差异,在3166~3540 cm-1处出现的吸收峰,代表O—H键的伸缩振动吸收峰。在870 cm-1处观察到的谱带归因于H2O的弯曲振动吸收峰。此外,N—H和C—N的特征峰分别出现在波长1510和1408 cm-1处,表明氨基酸诱导的Ca-P涂层中可能成功引入了苯丙氨酸、甲硫氨酸和天冬酰胺。同时,图中出现了Ca-P涂层的一般特征吸收峰。H2PO
图5
图5
AZ31镁合金表面Ca-P涂层和3种氨基酸诱导Ca-P涂层的Fourier红外光谱(FTIR)
Fig.5
FTIR of Ca-P coating (a), Ca-PPhe coating (b), Ca-PMet coating (c), and Ca-PAsn coating (d) on AZ31 magnesium alloy surface
图6
图6
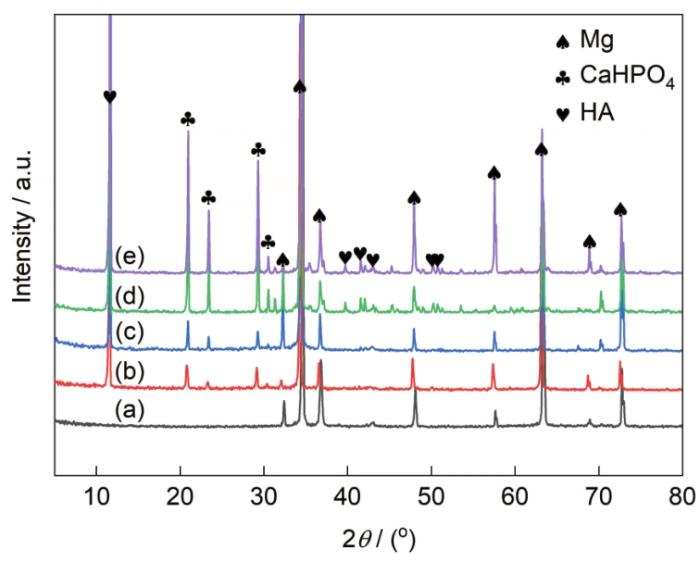
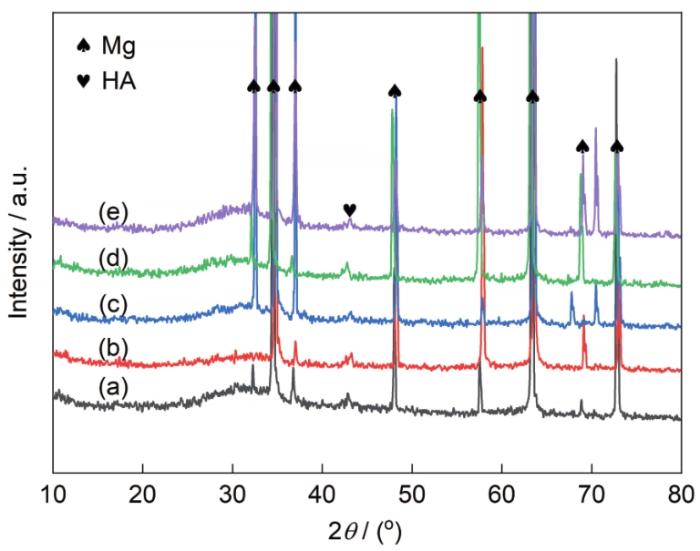
AZ31镁合金及其Ca-P涂层和3种氨基酸诱导Ca-P涂层的XRD谱
Fig.6
XRD spectra of AZ31 Mg alloy (a), Ca-P coating (b), Ca-PPhe coating (c), Ca-PMet coating (d), and Ca-PAsn coating (e) (HA—Ca10(PO4)6(OH)2)
2.2 涂层的耐蚀性能
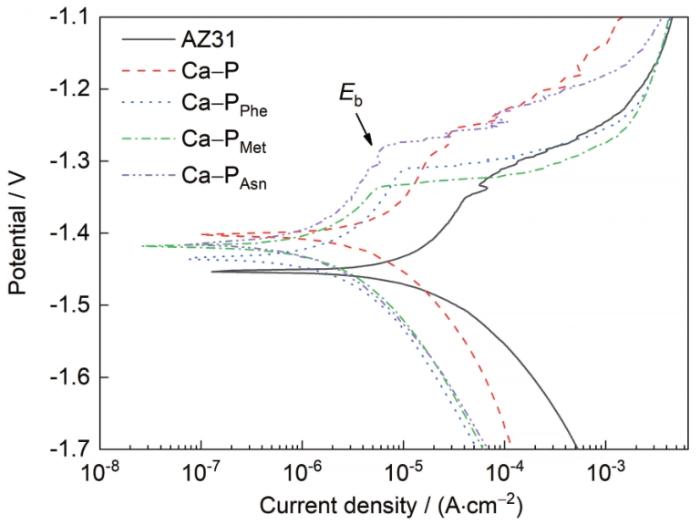
AZ31镁合金及其Ca-P涂层和3种氨基酸诱导Ca-P涂层的极化曲线如图7所示。用Tafel曲线外推法得到的Ecorr和icorr的拟合结果见表2。事实上,Ca-PPhe/Met/Asn涂层的icorr均比AZ31镁合金的icorr (1.73 × 10-5 A/cm2)小一个数量级,说明添加氨基酸使Ca-P涂层的耐蚀性能有明显提高。5种样品的icorr按以下顺序排列:AZ31镁合金(1.73 × 10-5 A/cm2) > Ca-P涂层(6.54 × 10-6 A/cm2) > Ca-PPhe涂层(3.80 × 10-6 A/cm2) > Ca-PMet涂层(2.99 × 10-6 A/cm2) > Ca-PAsn涂层(1.36 × 10-6 A/cm2)。从腐蚀动力学角度来说,Ca-PAsn涂层具有最佳的耐蚀性能。此外,Ca-PAsn涂层的破钝电位(Eb)比Ca-PPhe涂层和Ca-PMet涂层高。这进一步说明,Ca-PAsn涂层对镁合金具有更好的保护作用。通常来说,Rp的倒数与镁合金的腐蚀速率成正比[29]。5种样品的Rp可以按以下顺序排列:AZ31镁合金(2.14 × 106 Ω·cm2) < Ca-P涂层(8.06 × 106 Ω·cm2) < Ca-PPhe涂层(18.29 × 106 Ω·cm2) < Ca-PMet涂层(19.89 × 106 Ω·cm2) < Ca-PAsn涂层(20.16 × 106 Ω·cm2),从而进一步证明了Ca-PAsn涂层具有最佳的耐蚀性。
图 7
图 7
AZ31镁合金及其Ca-P涂层和3种氨基酸诱导Ca-P涂层的极化曲线
Fig.7
Polarization curves of AZ31Mg alloy, Ca-P coating, Ca-PPhe coating, Ca-PMet coating, and Ca-PAsn coating (Eb—broken shield potential)
表2 AZ31 镁合金及其Ca-P 涂层和3种氨基酸诱导Ca-P涂层的Tafel极化曲线拟合数据
Table 2
| Sample | Ecorr / mVSCE | icorr / (10-6 A·cm-2) | βa / (mV·dec-1) | βc / (mV·dec-1) | Rp / (106 Ω·cm2) |
|---|---|---|---|---|---|
| AZ31 | -1481.47 | 17.30 | 258.19 | -127.67 | 2.14 |
| Ca-P | -1402.11 | 6.54 | 257.40 | -230.00 | 8.06 |
| Ca-PPhe | -1435.36 | 3.80 | 387.58 | -271.98 | 18.29 |
| Ca-PMet | -1423.59 | 2.99 | 315.89 | -241.74 | 19.89 |
| Ca-PAsn | -1416.23 | 1.36 | 145.42 | -111.57 | 20.16 |
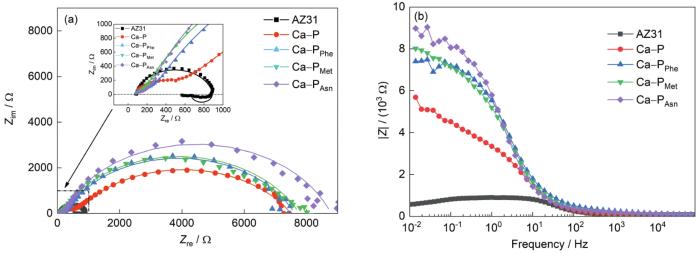
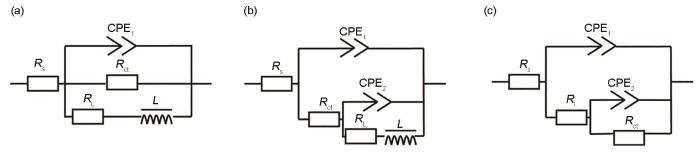
通过对AZ31镁合金基体、Ca-P涂层及3种氨基酸诱导Ca-P涂层样品在Hank's溶液中进行EIS测试,进一步研究Ca-PPhe、Ca-PMet和Ca-PAsn涂层的耐蚀性能,如图8所示。在等效电路图(图9)中,Rs表示溶液电阻;CPE1和CPE2表示常相位角元件;Rct表示电荷转移电阻;RL和L分别表示电感电阻和电感;R1表示涂层电阻。从图8a可见,AZ31镁合金和Ca-P涂层的Nyquist曲线由中高频电容环和低频电感环组成,其中中高频电容环通常归因于电荷转移(Rct)[30,31],一般来说,电荷转移电阻越大样品的溶解速率越慢,即Rct越高,耐蚀性能越好[32];而低频感应环(RL和L)则与α-Mg的溶解[33,34]和点蚀的发生有关。从EIS拟合数据(表3)中可以看出,AZ31基体和Ca-P涂层的RL分别为231.5和6856.0 Ω·cm2,表明与AZ31基体相比Ca-P涂层的点蚀程度更严重,可能是由于Ca-P涂层结构疏松多孔,导致腐蚀介质通过孔隙深入到基体,从而发生腐蚀。而3种氨基酸诱导的Ca-P涂层的低频区没有感抗的出现,进一步说明氨基酸的加入对Ca-P涂层点蚀的发生起一定的阻碍作用。同时,Rct按以下顺序从小到大排列:AZ31镁合金(578.5 Ω·cm2) < Ca-P涂层(593.8 Ω·cm2) < Ca-PPhe涂层(7266.0 Ω·cm2) < Ca-PMet涂层(7465.0 Ω·cm2) < Ca-PAsn涂层(8552.0 Ω·cm2),这说明Ca-PAsn涂层具有最佳的耐蚀性。此外,从图8a可以得出,Ca-PAsn涂层的曲率半径最大,也表明其具有最佳的耐蚀性能[35]。除此之外,在图8b中Ca-PAsn涂层的阻抗模量(|Z|0.01Hz)是最大的,也表明Ca-PAsn涂层的耐蚀性能最佳[20]。
图8
图8
AZ31 镁合金及其Ca-P涂层和3种氨基酸诱导Ca-P涂层的EIS分析
Fig.8
Nyquist (a) and Bode (b) curves of AZ31 Mg alloy, Ca-P coating, Ca-PPhe coating, Ca-PMet coating, and Ca-PAsn coating (Zim—imaginary part of impedance, Zre—real part of impedance, |Z|—impedance modulus)
图9
图9
AZ31镁合金及其Ca-P涂层和3种氨基酸诱导Ca-P涂层EIS的等效电路
Fig.9
Equivalent circuits of AZ31 Mg alloy (a), Ca-P coating (b), and Ca-PPhe/Met/Asn coating (c) (Rs—solution resistance, CPE—constant phase element, Rct—charge transfer resistance, L—equivalent inductance, RL—inductive resistance, R1—coating resistance)
表3 AZ31镁合金、Ca-P涂层和3种氨基酸诱导Ca-P涂层的EIS拟合数据
Table 3
| Sample | Rs | CPE1 | n1 | R1 | CPE2 | n2 | Rct | L | RL |
|---|---|---|---|---|---|---|---|---|---|
| Ω·cm2 | 10-5 Ω-1·s | kΩ·cm2 | 10-5 Ω-1·s | Ω·cm2 | H·cm2 | Ω·cm2 | |||
| AZ31 | 85.88 | 1.278 | 0.9056 | - | - | - | 578.5 | 439.7 | 231.5 |
| Ca-P | 57.25 | 0.136 | 0.6946 | - | 2.616 | 0.6438 | 593.8 | 3.7 | 6856.0 |
| Ca-PPhe | 70.00 | 0.875 | 0.6515 | 289.6 | 1.582 | 0.7757 | 7266.0 | - | - |
| Ca-PMet | 84.64 | 1.859 | 0.6809 | 226.3 | 1.693 | 0.8297 | 7465.0 | - | - |
| Ca-PAsn | 80.93 | 0.742 | 0.6782 | 157.8 | 1.873 | 0.8144 | 8552.0 | - | - |
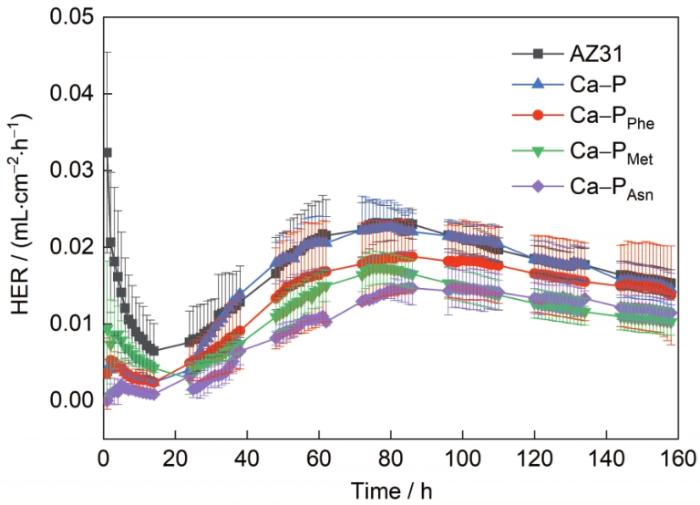
图10为AZ31镁合金、Ca-P涂层和3种氨基酸诱导Ca-P涂层的析氢测试结果。在浸泡前期,由于样品表面氧化膜的形成或表面涂层的存在[36],样品的析氢速率急速下降。经过24 h的浸泡后,析氢速率呈现出逐渐上升的状态,这是由于Ca-P涂层不致密,溶液中腐蚀性介质通过孔隙到达镁合金基体,进而导致腐蚀加快。经过80 h左右的浸泡,析氢速率逐渐下降并趋于平稳,推测可能是磷酸盐沉淀类腐蚀产物的生成对样品产生二次保护。析氢测试结果显示,Ca-P涂层在模拟人体体液(Hank's)中的长期耐蚀性能优于AZ31基体,而氨基酸诱导Ca-P涂层的耐蚀性优于Ca-P涂层,其中Ca-PAsn涂层的析氢速率最低。析氢测试结果与电化学极化和EIS测试结果相吻合。因此,3种氨基酸诱导的Ca-P涂层中Ca-PAsn涂层具有最佳的耐蚀性。
图10
图10
AZ31镁合金、Ca-P涂层和3种氨基酸诱导Ca-P涂层的析氢曲线
Fig.10
Hydrogen evolution rate (HER) curves of AZ31Mg alloy, Ca-P coating, Ca-PPhe coating, Ca-PMet coating, and Ca-PAsn coating
2.3 涂层的腐蚀机理
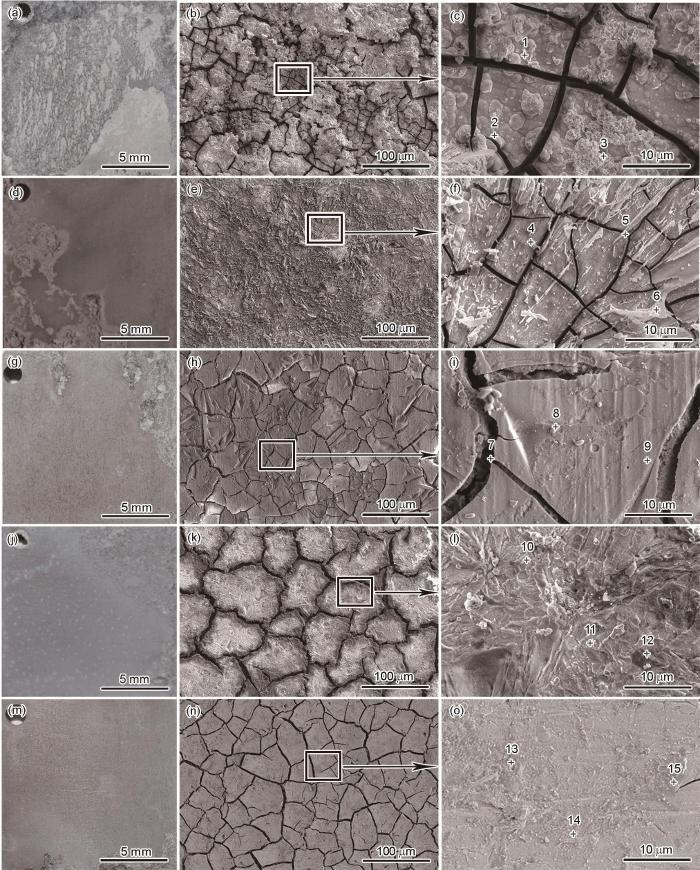
图11为样品经过160 h浸泡后的宏观形貌和SEM像。可以看出,对于AZ31基体(图11a~c),样品表面出现了明显的腐蚀坑及较多的裂纹和腐蚀产物。EDS分析(表4)表明,AZ31基体中Mg元素的含量较高,而Ca和P元素含量较低,进一步说明AZ31基体遭受到了严重的腐蚀。Ca-P涂层(图11d~f)经过浸泡后,涂层基本遭到破坏,产生了较为明显的裂纹。Ca-PPhe涂层(图11g~i)经过浸泡实验后,样品四周出现明显的腐蚀坑,并且产生较深的腐蚀裂纹。Ca-PMet涂层(图11j~l)可以观察到一些微小的裂纹和腐蚀产物,表明涂层遭受了一定的腐蚀。而Ca-PAsn涂层(图11m~o)浸泡160 h后涂层结构基本保持完整,没有出现较为明显的腐蚀现象。从图中可以看出,样品表面占主导地位的元素有:C、O、N、Mg、Ca和P。此外,AZ31样品中存在Ca和P是由于Hank's溶液中含有Ca和P元素。从3种氨基酸诱导的Ca-P涂层浸泡160 h后的元素分布来看,附着在样品表面的腐蚀产物中不仅有Mg(OH)2还有钙磷产物。对比3种氨基酸诱导的Ca-P涂层,Ca-PAsn涂层中的Ca和P元素依然保持着很高的含量,Mg元素的含量也很低。这可以推测出经过浸泡实验后样品表面的涂层保持着良好的完整性。
图11
图11
AZ31镁合金、Ca-P涂层和3种氨基酸诱导Ca-P涂层浸泡160 h后的宏观形貌和SEM像
Fig.11
Digital camera photographs (a, d, g, j, m) and SEM images (b, c, e, f, h, i, k, l, n, o) after 160 h immersion for AZ31 Mg alloy (a-c), Ca-P coating (d-f), Ca-PPhe coating (g-i), Ca-PMet coating (j-l), and Ca-PAsn coating (m-o)
表4 图11中点1~15的EDS分析结果 (atomic fraction / %)
Table 4
| Point | C | N | O | Mg | Ca | P |
|---|---|---|---|---|---|---|
| 1 | 3.98 | 1.45 | 63.07 | 14.16 | 8.72 | 8.61 |
| 2 | 6.02 | 1.09 | 62.60 | 15.06 | 7.62 | 7.60 |
| 3 | 5.79 | 0.93 | 70.34 | 10.79 | 5.20 | 6.95 |
| 4 | 8.20 | 1.91 | 68.69 | 5.62 | 7.70 | 7.88 |
| 5 | 5.75 | 1.45 | 70.74 | 1.33 | 9.04 | 11.68 |
| 6 | 5.29 | 1.32 | 65.10 | 3.51 | 15.99 | 8.79 |
| 7 | 3.42 | 1.31 | 61.38 | 5.99 | 13.20 | 14.69 |
| 8 | 11.40 | 4.92 | 61.09 | 3.52 | 10.20 | 8.87 |
| 9 | 12.38 | 5.05 | 58.08 | 2.51 | 11.36 | 10.62 |
| 10 | 6.45 | 1.86 | 63.70 | 3.46 | 13.96 | 10.57 |
| 11 | 6.96 | 1.97 | 70.64 | 2.69 | 10.55 | 7.19 |
| 12 | 5.61 | 1.87 | 62.78 | 3.33 | 14.22 | 12.18 |
| 13 | 5.36 | 1.65 | 60.92 | 3.70 | 15.83 | 12.97 |
| 14 | 4.92 | 1.73 | 60.67 | 3.93 | 16.60 | 12.15 |
| 15 | 4.84 | 1.46 | 61.37 | 2.42 | 14.84 | 15.07 |
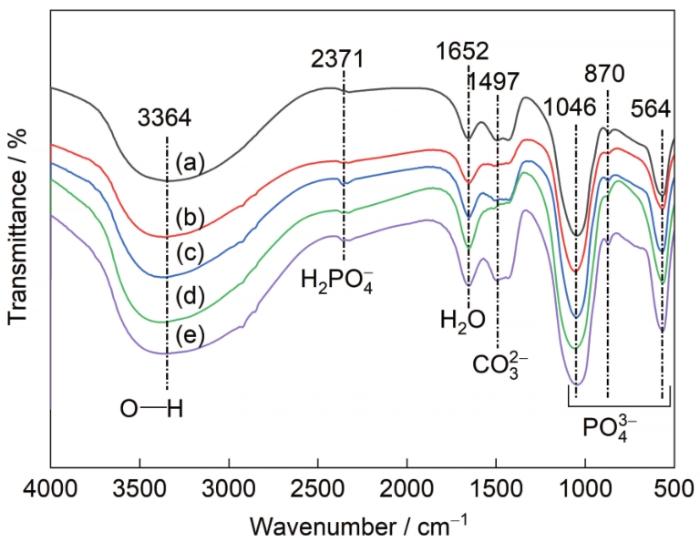
图12为样品经过160 h浸泡实验后的FTIR。对比浸泡前的FTIR (图5),浸泡后的样品变化主要表现为氨基酸的官能团(N—H、C—N)的消失。在3384 cm-1处的峰位来源于O—H的拉伸振动[37],其对应于化合物中的结晶水。2371 cm-1处代表H2PO
图12
图12
AZ31镁合金及其Ca-P涂层和3种氨基酸诱导Ca-P涂层浸泡160 h后的FTIR
Fig.12
FTIR of AZ31 Mg alloy (a), Ca-P coating (b), Ca-PPhe coating (c), Ca-PMet coating (d), and Ca-PAsn coating (e) immersed in Hank's solution after 160 h
图13
图13
AZ31镁合金及其Ca-P涂层和3种氨基酸诱导Ca-P涂层浸泡160 h后的XRD谱
Fig.13
XRD spectra of AZ31 Mg alloy (a), Ca-P coating (b), Ca-PPhe coating (c), Ca-PMet coating (d), and Ca-PAsn coating (e) immersed in Hank's solution after 160 h
在模拟人体体液(Hank's)中含有Cl-、Ca2+和H2PO
3 分析与讨论
3.1 氨基酸的吸附作用
耐蚀性能问题是镁合金应用过程中的一个关键性问题,可以通过添加缓蚀剂来解决。添加缓蚀剂作为一种常用的腐蚀防护手段,由于其成本低、易于操作而得到广泛应用。但缓蚀剂的溶解可能会对人类和环境产生损害,因此,在保护镁合金表面不受腐蚀的同时,应使用高效、环保、可降解的绿色型缓蚀剂,来起到阻挡腐蚀物质进入的作用。
氨基酸诱导Ca-P涂层晶粒呈现出均匀的径向晶粒,表明氨基酸的加入可以降低气孔率,减小晶粒粗大引起的涂层不均匀性。电化学结果表明,氨基酸诱导Ca-P涂层的腐蚀电流密度比AZ31镁合金基体降低了一个数量级,其中3种氨基酸的吸附效果顺序为天冬酰胺>甲硫氨酸>苯丙氨酸。经过长时间的浸泡实验,含有氨基酸的Ca-P涂层表面腐蚀损伤较小,这也证明了它们有效提高了镁合金的耐蚀性能。3种氨基酸中的极性官能团优先吸附在镁合金表面,阻止溶液中腐蚀性离子的入侵,同时,羧基与溶液中游离Ca2+和Mg2+发生反应,溶液中的Ca2+、HPO
基于上述事实,氨基酸及其相关化合物对镁合金的腐蚀具有很好的抑制效果。在各种缓蚀剂中氨基酸被认为是许多金属的绿色缓蚀剂,其与大多数有机缓蚀剂类似,这些化合物的抑制效果归因于氨基酸分子通过“直接”或“间接”吸附在镁合金表面而积聚,从而减少镁合金与溶液中腐蚀介质的接触。氨基酸吸附能力的大小受其表面电荷、化学性质和官能团的影响。通常,氨基酸吸附方式分为2种:(1) 氨基酸的官能团可以通过化学吸附机制吸附在镁合金表面;(2) 带电的官能团可以与带电镁合金表面或带电粒子发生静电相互作用。由此可知,氨基酸和镁合金表面之间的吸附效果[41,42]主要取决于官能团。氨基酸含有2个化学性质相反的官能团,既含有具有酸性的羧基,又含有具有碱性的氨基,因而,氨基酸是两性电解质,它能通过氨基的N原子[43]和羧基的O原子[44]与金属的d轨道配合,增强吸附效果。此外,氨基酸的吸附作用也受含孤对电子的杂原子等因素的影响,杂原子可以与镁合金基体的空位分子轨道共享其孤对电子[45],从而具有较好的吸附效果。
3.2 成膜机理
AZ31镁合金中存在的α-Mg相优先与H2O分子发生反应,产生Mg2+、OH-和H2:
与此同时溶液中的H2PO
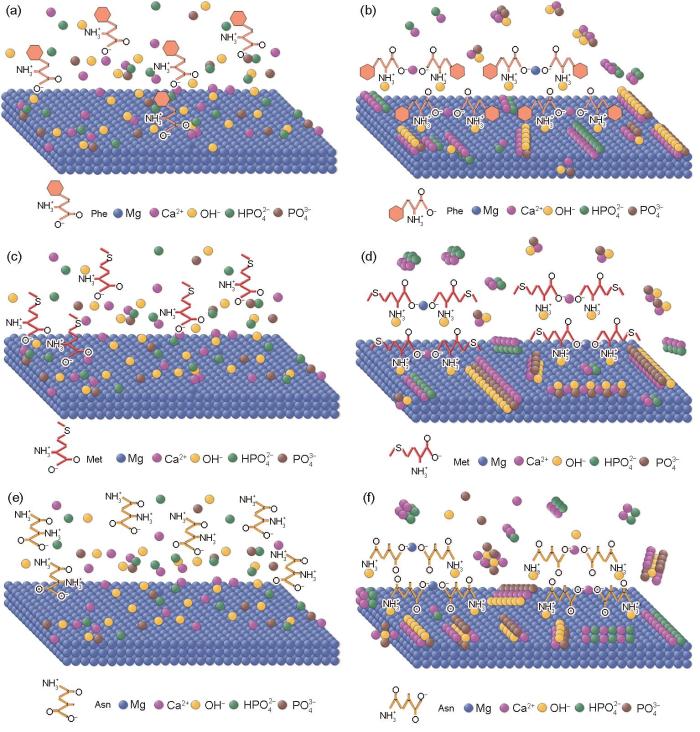
苯丙氨酸含有非极性苯环、极性基团氨基和羧基,极性基团能吸附在镁合金表面成膜;亲水的羧基带有较多的负电荷,易与溶液中的Ca2+和Mg2+发生螯合反应形成稳定致密的化学吸附保护膜;憎水的苯环向上形成定向排列,使得腐蚀介质与镁合金表面隔开,从而抑制腐蚀的发生。如图14a和b所示。
图14
图14
Ca-PPhe、Ca-PMet和Ca-PAsn涂层的形成机理示意图
Fig.14
Schematics of the formation mechanisms of Ca-PPhe (a, b), Ca-PMet (c, d), and Ca-PAsn (e, f) coatings
甲硫氨酸可以依靠其特有的S活性中心吸附到镁合金表面起到保护基体的作用。溶液中的Ca2+与镁合金表面吸附的羧基形成保护性络合物,进一步抑制镁合金基体的腐蚀。如图14c和d所示。
与其他氨基酸相比,天冬酰胺则有更多的N原子吸附在镁合金表面来阻止腐蚀的发生,羧基与溶液中游离Ca2+和Mg2+发生反应。同时,溶液中的HPO
4 结论
(1) 3种氨基酸诱导的Ca-P涂层的物相主要为HA和CaHPO4,但随着在Hank's溶液浸泡时间的延长,氨基酸诱导的Ca-P涂层也遭到部分破坏,出现CaHPO4峰消失和HA峰减少的现象。与Ca-P涂层相比,3种氨基酸有机添加剂均能够不同程度地增大Ca-P涂层的厚度,并且氨基酸诱导的Ca-P涂层更为致密,且呈现出规则的荷叶状晶体。其中天冬酰胺的加入明显增加了Ca-PAsn涂层的厚度和致密性。
(2) 动电位极化曲线和EIS结果均表明,3种氨基酸诱导的Ca-P涂层的耐蚀性能较AZ31镁合金基体及Ca-P涂层均有所提高,相对来说,天冬酰胺的加入对Ca-P涂层耐蚀性能的提高最为明显。
(3) 氨基酸促进Ca-P涂层成膜的机理通常归因于其对金属离子的吸附,即氨基酸中的极性官能团优先吸附在镁合金表面,与Ca2+作用形成中间物,羧基与溶液中游离Ca2+和Mg2+发生反应,同时溶液中的Ca2+、HPO
参考文献
Biomedical magnesium alloys: Composition, microstructure and corrosion
[J].
医用镁合金: 成分、组织及腐蚀
[J].
Effect of second phases on the corrosion behaviour of wrought Mg-Zn-Y-Zr alloy
[J].
Research progress in biodegradable metals for stent application
[J].
血管支架用可降解金属研究进展
[J].
Dealloying corrosion of anodic and nanometric Mg41Nd5 in solid solution-treated Mg-3Nd-1Li-0.2Zn alloy
[J].
Recent development of self-healing coating on magnesium alloys: A review
[J].
镁合金表面自愈合涂层进展
[J].
Advances in coatings on biodegradable magnesium alloys
[J]. J.
Corrosion resistance and electrical conductivity of a nano ATO-doped MAO/methyltrimethoxysilane composite coating on magnesium alloy AZ31
[J].
Hybrid coatings with collagen and chitosan for improved bioactivity of Mg alloys
[J].
Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review
[J].
Enhancing biocompatibility and corrosion resistance of biodegradable Mg-Zn-Y-Nd alloy by preparing PDA/HA coating for potential application of cardiovascular biomaterials
[J].
Revisiting the cracking of chemical conversion coating on magnesium alloys
[J].
Research advances of layered double hydroxides coatings on biomedical metals
[J].
生物医用金属表面水滑石涂层的研究进展
[J].
Biocorrosion behavior of micro-arc-oxidized AZ31 magnesium alloy in different simulated dynamic physiological environments
[J].
Biodegradation behavior of micro-arc oxidation coating on magnesium alloy—From a protein perspective
[J].
Study on graphene modified organic anti-corrosion coatings: A comprehensive review
[J]. J.
Biodegradation, hemocompatibility and covalent bonding mechanism of electrografting polyethylacrylate coating on Mg alloy for cardiovascular stent
[J].
Hybrid sol-gel coatings based on silanes-amino acids for corrosion protection of AZ91 magnesium alloy: Electrochemical and DFT insights
[J].
Influences of protein adsorption on the in vitro corrosion of biomedical metals
[J].
蛋白质吸附对医用金属材料体外腐蚀行为的影响
[J].
The corrosion inhibition action of five amino acid compounds on copper in HCl solution
[J].
五种氨基酸在HCl溶液中对铜的缓蚀作用
[J].
Amino acid interleaved layered double hydroxides as promising hybrid materials for AA2024 corrosion inhibition
[J].
Experimental evaluation of inhibition performance and inhibition mechanism analysis of amino acid inhibitors
[J].
氨基酸缓蚀剂缓蚀性能的实验评价与缓蚀机制分析
[J].
The use of cysteine, N-acetyl cysteine and methionine as environmentally friendly corrosion inhibitors for Cu-10Al-5Ni alloy in neutral chloride solutions
[J].
Synergistic effect of SAMs of S-containing amino acids and surfactant on corrosion inhibition of 316L stainless steel in 0.5 M NaCl solution
[J].
In vitro corrosion of pure Mg in phosphate buffer solution—Influences of isoelectric point and molecular structure of amino acids
[J].
Corrosion resistance of an amino acid-bioinspired calcium phosphate coating on magnesium alloy AZ31
[J].
The effect of small-molecule bio-relevant organic components at low concentration on the corrosion of commercially pure Mg and Mg-0.8Ca alloy: An overall perspective
[J].
Improving the corrosion resistance of ZEK100 magnesium alloy by combining high-pressure torsion technology with hydroxyapatite coating
[J].
In vitro corrosion resistance of layer-by-layer assembled polyacrylic acid multilayers induced Ca-P coating on magnesium alloy AZ31
[J].
Designing for the chemical conversion coating with high corrosion resistance and low electrical contact resistance on AZ91D magnesium alloy
[J].
Permanganate conversion coating on AZ31 magnesium alloys with enhanced corrosion resistance
[J].
Corrosion resistance of in-situ growth of nano-sized Mg(OH)2 on micro-arc oxidized magnesium alloy AZ31—Influence of EDTA
[J].
Study of the corrosion product films formed on the surface of Mg-xZn alloys in NaCl solution
[J].
On the corrosion mechanism of Mg investigated by electrochemical impedance spectroscopy
[J].
Enhanced corrosion resistance and biocompatibility of biodegradable magnesium alloy modified by calcium phosphate/collagen coating
[J].
The effects of a phytic acid/calcium ion conversion coating on the corrosion behavior and osteoinductivity of a magnesium-strontium alloy
[J].
In vitro degradation of pure magnesium—The synergetic influences of glucose and albumin
[J].
In vitro corrosion resistance and antibacterial performance of novel tin dioxide-doped calcium phosphate coating on degradable Mg-1Li-1Ca alloy
[J].
Bioactive and anti-corrosive bio-MOF-1 coating on magnesium alloy for bone repair application
[J]. J.
Corrosion resistance of self-cleaning silane/polypropylene composite coatings on magnesium alloy AZ31
[J].
Enhanced corrosion resistance and biocompatibility of polydopamine/dicalcium phosphate dihydrate/collagen composite coating on magnesium alloy for orthopedic applications
[J]. J.
Adsorption of arginine, glycine and aspartic acid on Mg and Mg-based alloy surfaces: A first-principles study
[J].
An effective hybrid organic/inorganic inhibitor for alkaline aluminum-air fuel cells
[J].
Amino acid and TiO2 nanoparticles mixture inserted into sol-gel coatings: An efficient corrosion protection system for AZ91 magnesium alloy
[J].
A DFT study of the adsorption of short peptides on Mg and Mg-based alloy surfaces
[J].
Effect of amino acids and montmorillonite nanoparticles on improving the corrosion protection characteristics of hybrid sol-gel coating applied on AZ91 Mg alloy
[J].