铅冷快堆(lead-cooled fast reactor,LFR)是第四代先进核能系统中最具发展潜力的反应堆型,LFR的冷却剂为纯Pb或Pb-Bi共晶(lead-bismuth eutectic,LBE)合金。与纯Pb相比,LBE (Pb∶Bi = 44.5∶55.5,质量比)能够使反应堆的运行温度拓展到更广范围[1],但同时,高温高密度的LBE加剧了对结构材料的腐蚀。为了实现LFR的高质量建设,亟须研制兼顾高温强度、耐LBE腐蚀与抗辐照肿胀等特点的包壳及结构材料。
国内外开展了大量核用材料如奥氏体不锈钢(如316L)、铁素体/马氏体不锈钢(如T91)、SiC复合材料(如β-SiC)等耐LBE腐蚀行为的研究。结果表明,奥氏体不锈钢由于添加较高含量的Ni,在500~560 ℃的LBE环境中表现出较高的溶解性。T91钢的耐溶解性比316L钢略好,但是长时间腐蚀后仍然观察到LBE沿T91钢的晶界发生渗透,局部腐蚀严重[2,3]。随着先进反应堆服役温度的提升,奥氏体不锈钢和铁素体/马氏体不锈钢已经无法应用于550 ℃以上的Pb-Bi环境。至于SiC陶瓷,Stepanov等[4]报道了由SiC + MeO构成的摩擦副在400~550 ℃、(1~4) × 10-6% (质量分数,下同)氧浓度的LBE环境中具有较好的耐腐蚀性能,但是,SiC包壳管与结构材料的加工成型较困难。即使将SiC制成涂层使用,其与基体之间较大的热膨胀系数差异也使得SiC涂层发生开裂甚至剥离,进而发生LBE对基体的侵蚀[5]。
氧化物弥散强化(oxide dispersion strengthened,ODS)合金因其材料内部弥散分布着纳米尺度的氧化物颗粒(Y-Ti-O、Y-Al-O和/或Y-Zr-O相)钉扎位错与晶界、捕获辐照产生的He泡与离位原子等而表现出优异的高温力学性能和杰出的抗辐照肿胀性能[6]。同时,ODS合金具有较好的加工成型能力,已成功轧制出壁厚为0.3~0.5 mm的包壳管[7,8],是第四代先进反应堆燃料包壳材料的候选材料。相对于上述材料体系,对ODS合金与LBE的相容性研究有限。Takaya等[9]研究了Al含量为0~3.5% (质量分数,下同)、Cr含量为12%~17%的铁基ODS合金在550和650 ℃、10-6%和10-8%氧浓度LBE中的腐蚀行为,结果表明,未添加Al元素的ODS FeCr合金在550 ℃时发生了严重的晶界腐蚀,单纯依靠增加Cr含量已经无法保护合金基体。将Al含量增加到3.5%时,致密的Al2O3薄膜生成,阻止了LBE对合金基体的进一步侵蚀。基于保护性Al2O3薄膜的生成,本团队的前期工作[10]也证明了Fe-13.5Cr-4Al-0.35Y2O3 (质量分数,%,下同)合金在550 ℃、饱和氧浓度(约1.17 × 10-3%) LBE熔液中具有优异的耐腐蚀性能,并将兼具优异高温力学性能与耐LBE腐蚀性能的ODS FeCrAl合金用于包壳管材的制备。
对于ODS合金,Al元素的添加促进了合金表面Al2O3薄膜的生成,同时也促进合金基体中Y-Al-O强化相的生成。与ODS FeCr合金中的Y-Ti-O相纳米团簇相比,Y-Al-O相颗粒尺寸略大,使ODS FeCrAl合金的强度略有降低。为弥补Al元素添加导致的力学性能降低,目前公认的措施是在合金体系中添加微量Zr元素,利用尺寸更细小、与基体晶格错配度更低的Y-Zr-O相替代Y-Al-O相纳米颗粒优先析出的特点[6,11],实现ODS FeCrAl合金高温强度、热稳定性及抗辐照性能的提升。然而,针对高强度的含Zr ODS FeCrAl合金耐LBE腐蚀性能的研究较少。因此,本工作以一种含Zr (13.5~14)Cr-ODS FeCrAl合金为研究对象,开展其在550 ℃、静态饱和氧LBE熔液中的腐蚀实验,分析合金基体中Zr、Ti、Al和O等微量元素对氧化产物及氧化膜致密性的影响,揭示添加Zr元素的ODS FeCrAl合金在LBE中生成保护性Al2O3薄膜的关键影响因素。
1 实验方法
1.1 合金试样制备
表1 氧化物弥散强化(ODS) FeCrAl合金的化学成分 (mass fraction / %)
Table 1
| Alloy | Cr | W | Al | Ti | Zr | Y | O | Ex.O | Fe |
|---|---|---|---|---|---|---|---|---|---|
| CAZ-1 | 13.48 | 1.69 | 5.30 | - | 0.31 | 0.36 | 0.12 | 0.023 | Bal. |
| CAZ-2 | 13.58 | 1.69 | 5.28 | - | 0.31 | 0.36 | 0.24 | 0.143 | Bal. |
| CAZ-3 | 13.51 | 1.70 | 5.23 | 0.35 | 0.30 | 0.37 | 0.23 | 0.130 | Bal. |
| CAZ-4 | 13.99 | 1.81 | 4.11 | 0.33 | 0.28 | 0.35 | 0.10 | 0.006 | Bal. |
| CAZ-5 | 13.99 | 1.81 | 4.18 | 0.35 | 0.29 | 0.34 | 0.23 | 0.138 | Bal. |
Note: Ex.O (i.e., excess O content) is equal to the O content of ODS alloy minus the O content provided by Y2O3 [
1.2 Pb-Bi腐蚀实验
沿ODS FeCrAl合金棒料的轴向方向,利用电火花线切割设备将棒料切割成直径12 mm、厚5 mm的试样。在靠近边缘处钻出直径1 mm的孔洞后,试样经砂纸打磨、金刚石研磨膏抛光,然后使用石油醚和酒精清洗、吹干,备用。
腐蚀实验在550 ℃静态饱和氧的LBE中进行,试样采用悬挂方式浸泡在腐蚀熔液中,腐蚀时间为10000 h。LBE实验装置详见文献[14]。
1.3 试样表征
腐蚀实验前,在ODS FeCrAl合金棒料上随机取样,经薄片切割、打磨、冲孔和化学双喷制成直径3 mm的透射电子显微镜(TEM)试样,利用F200C型TEM观察纳米氧化物形貌;利用高分辨透射电镜(HRTEM)模式获得纳米氧化物的相位衬度图,每组ODS合金的氧化物颗粒数量控制在85~95个,并通过快速Fourier变换(FFT)解析纳米氧化物的晶体结构,进而确定Y-Al-O和Y-Zr-O相纳米颗粒在总析出氧化物颗粒中的数量占比。同时,沿平行于合金棒料轴向方向切割试样,经磨抛后使用FeCl3溶液(2.5 g FeCl3 + 50 mL HCl + 100 mL去离子水)进行金相腐蚀,利用Axio Observer Z1光学显微镜(OM)观察合金的显微组织。
腐蚀实验后,采用Merlin Compact型场发射扫描电子显微镜(SEM)观察氧化产物的截面形貌,利用SEM附带的能谱仪(EDS)、1600型电子探针(EPMA)分析氧化膜截面的元素分布与化学组成。使用体积比为1∶1∶1的CH3COOH + H2O2 + C2H5OH溶液去除试样表面残留的LBE后,采用SEM进行表面形貌观察和成分分析。使用D/max-2400型X射线衍射仪(XRD)分析腐蚀产物成分,Co靶(波长λ = 0.179801 nm),步长0.02°。
2 实验结果
2.1 含Zr ODS FeCrAl合金的微观结构
图1
图1
含Zr ODS FeCrAl合金中纳米氧化物形貌的TEM明场像与颗粒尺寸分布
Fig.1
TEM bright field images (a1-e1) and corresponding particle size distributions (a2-e2) of oxide nanoparticles in ODS FeCrAl alloys containing Zr
(a1, a2) CAZ-1 (b1, b2) CAZ-2 (c1, c2) CAZ-3 (d1, d2) CAZ-4 (e1, e2) CAZ-5
如前所述,Zr元素添加到ODS FeCrAl合金中,会优先析出尺寸较为细小的Y-Zr-O相纳米颗粒,同时研究[15,16]表明,Y-Al-O和Y-Zr-O相纳米颗粒的组成和数量占比依赖于合金元素与O含量。考虑到本工作合金的Al、Ti和O含量不同,腐蚀实验前先通过解析纳米氧化物的晶体结构来辨别每种合金试样内纳米强化相的组成,结果也列于表2,该结果可反映出纳米氧化物的组成随合金元素和O含量变化的趋势。很明显,合金的O含量增加(即:CAZ-1合金与CAZ-2合金的比较、 CAZ-4合金与CAZ-5合金的比较),可以使得合金内Y-Zr-O相纳米颗粒的析出数量增加,Y-Al-O相纳米颗粒的析出数量降低,进而O含量较高的CAZ-2和CAZ-5合金内其纳米强化相的平均颗粒尺寸降低。O含量过低时(即CAZ-4,Ex.O = 0.006%),合金内还生成了少量纳米尺寸的ZrO2颗粒。值得注意的是,含Zr ODS FeCrAl合金中添加Ti元素,合金基体中还弥散析出Y-Ti-O相纳米颗粒。相同的纳米氧化物组成在Dou等[17]报道的含Zr ODS FeCrAl合金中也出现过。对于CAZ-3合金,O含量较高时Ti元素的添加促进了尺寸细小的Y-Ti-O相纳米颗粒生成,与Y4Al2O9颗粒相比,Y-Ti-O析出相尺寸更加细化[18],进而使得颗粒体积密度增加。
图2为未进行LBE腐蚀的含Zr ODS FeCrAl合金试样平行于轴向方向上显微组织的OM像。由于采用了旋锻工艺对合金进行变形处理,冷加工细化了合金晶粒,并沿锻造方向拉长了晶粒。即使经过最后一道次850 ℃热处理,沿棒料轴向方向上仍然可以观察到大量拉长和变形的铁素体晶粒。
图2
图2
含Zr ODS FeCrAl合金轴向方向显微组织的OM像
Fig.2
OM images parallel to the axial of ODS FeCrAl alloy containing Zr
(a) CAZ-1 (b) CAZ-2 (c) CAZ-3 (d) CAZ-4 (e) CAZ-5
表2 含Zr ODS FeCrAl合金中纳米强化相物相分析结果
Table 2
| Alloy | d nm | ρv 1022 m-3 | Quantitative percentage of oxide nanoparticles with different components / % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Y4Al2O9 | YTiO3 | Y2TiO5 | Y4Zr3O12 | Y2Zr2O7 | YZrO3 | ZrO2 | |||
| CAZ-1 | 8.87 | 2.67 | 23.4 | 0 | 0 | 33.3 | 6.7 | 36.6 | 0 |
| CAZ-2 | 6.62 | 3.89 | 4.1 | 0 | 0 | 54.2 | 0 | 41.7 | 0 |
| CAZ-3 | 6.89 | 4.00 | 2.6 | 0 | 17.1 | 46.3 | 29.1 | 4.9 | 0 |
| CAZ-4 | 7.84 | 1.60 | 28.6 | 7.1 | 0 | 50.1 | 7.1 | 0 | 7.1 |
| CAZ-5 | 7.24 | 3.03 | 4.2 | 0 | 22.2 | 30.6 | 29.1 | 13.9 | 0 |
Note: The quantitative percentage values are obtained from fast Fourier transform (FFT) pattern recognition of HRTEM image of 85-95 oxide nanoparticles in each alloy specimen. d—average size, ρv—volume fraction
2.2 Ti含量对含Zr ODS FeCrAl合金氧化膜组成的影响
图3
图3
经550 ℃、饱和氧Pb-Bi共晶(LBE)腐蚀10000 h后CAZ-2合金表面氧化产物形貌的SEM像及EDS分析
Fig.3
Surface SEM images and EDS analyses of CAZ-2 alloy exposed to oxygen-saturated lead-bismuth eutectic (LBE) at 550 oC for 10000 h (wm—mass fraction, wa—atomic fraction)
(a) morphology at low magnification (b, c) morphologies of different oxides at high magnification and corresponding EDS results
图4
图4
经550 ℃、饱和氧LBE腐蚀10000 h后CAZ-3合金表面氧化产物形貌的SEM像及EDS分析
Fig.4
Surface SEM images and EDS analyses of CAZ-3 alloy exposed to oxygen-saturated LBE at 550 oC for 10000 h
(a) morphology at low magnification (b, c) morphologies of different oxides at high magnification and corresponding EDS results
图5
图5
经550 ℃、饱和氧LBE腐蚀10000 h后CAZ-2和CAZ-3合金氧化产物截面形貌的SEM像及基底氧化物EDS线扫描结果
Fig.5
Cross-sectional SEM images of corrosion products (a, c) and EDS line scanning results of basal oxide along line 1 in Fig.5a (b) and line 2 in Fig.5c (d) on CAZ-2 (a, b) and CAZ-3 (c, d) alloys exposed to oxygen-saturated LBE at 550 oC for 10000 h (Insets in Figs.5a and c show the locally enlarged images. IOZ—internal oxidation zone)
图6
图6
经550 ℃、饱和氧LBE腐蚀10000 h后CAZ-2和CAZ-3合金表面疖状腐蚀产物截面的EPMA元素面扫描结果
Fig.6
EPMA elemental mapping results of cross-section of oxide nodules on CAZ-2 (a) and CAZ-3 (b) alloys exposed to oxygen-saturated LBE at 550 oC for 10000 h
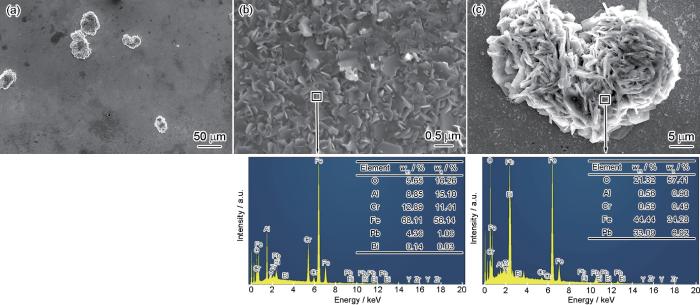
进一步地,统计了不同区域疖状氧化物的厚度,CAZ-2合金试样疖状氧化物的最大厚度可以达到24.1 μm,CAZ-3试样可达27.1 μm。尽管如此,其最大厚度仍然小于相同温度下5000 h、饱和氧LBE熔液腐蚀的AISI 316L (Fe-17Cr-12Ni-2Mo-1.8Mn-0.35Si)、MANET II (Fe-10Cr-0.7Ni-0.6Mo-0.8Mn-0.2V-0.1Nb-0.1C)和EP823 (Fe-12Cr-0.9Ni-0.7Mo-0.7Mn-0.4V-0.4Nb-1.2W-1.8Si-0.15C)不锈钢上多层腐蚀产物的厚度(≥ 50 μm)[21,22],同时,也小于相同温度、相同腐蚀时间的HT-9、T91和12Cr-Si不锈钢表面腐蚀产物的厚度(> 40 μm)[23]。由此表明,富Al、Cr的内部氧化层和IOZ在延缓氧化层向基体方向上延伸具有积极作用。同时,LBE沿着向外生长的Fe的氧化物的晶界向内扩散,到达外部氧化层和内部氧化层的分界处,即原始合金表面,但是被富Al、Cr氧化物的内部氧化层及IOZ所阻挡(见图6),表明富Al、Cr的内部氧化层和IOZ对抵挡LBE侵蚀也具有积极作用。
为获得腐蚀产物的确切组成,对去除LBE的试样表面进行XRD检测,采集的数据经归一化处理后,结果如图7所示。CAZ-2合金试样表面的疖状氧化物数量少,且Al2O3薄膜厚度小,因此只检测到合金基体的谱峰。其他几种合金表面可观察到立方结构的Al2O3 (PDF#03-0873)、立方结构的Fe3O4 (PDF#19-0629)、尖晶石结构的FeCr2O4 (PDF#34-0140)及Fe(Cr, Al)2O4 (PDF#03-0873)氧化物,另外还有少量的铅铁复合氧化物PbFe12O19 (PDF#72-0737)及PbFe6O10 (PDF#33-0757),这里表示为PbO·xFe2O3 (x为3或6)。结合图5和6可知,疖状氧化物的外部为Fe3O4,内部氧化层为FeCr2O4与Fe(Cr, Al)2O4。相似的氧化产物也出现在经400~600 ℃、10-6%氧浓度LBE腐蚀的Fe-(6~14)Cr-(4~8)Al合金表面[24]。
图7
图7
经550 ℃、饱和氧LBE腐蚀10000 h后5种合金的XRD谱
Fig.7
XRD spectra of ODS FeCrAl alloys exposed to oxygen-saturated LBE at 550 oC for 10000 h
2.3 Al含量对含Zr ODS FeCrAl合金氧化膜组成的影响
图8为CAZ-3 (Al含量5.23%)和CAZ-5 (Al含量4.18%)合金经550 ℃、饱和氧LBE腐蚀10000 h后的表面与截面形貌。与CAZ-3合金类似,CAZ-5合金表面除了生成Al2O3薄膜,也观察到数量较多的疖状氧化物。相对于CAZ-3合金上疖状氧化物在宽度上的扩展,CAZ-5试样的多层氧化物更倾向于向合金基体内部延伸。在多个视场下统计得到CAZ-3和CAZ-5合金疖状腐蚀产物的平均厚度分别为(20.97 ± 3.85)和(23.82 ± 3.72) μm,表明Al含量降低导致合金内氧化程度加剧,内部氧化层向基体深处扩展。
图8
图8
经550 ℃、饱和氧LBE腐蚀10000 h后CAZ-3和CAZ-5合金氧化产物表面与截面形貌的SEM像
Fig.8
Surface (a, c) and cross-sectional (b, d) SEM images of oxide products on CAZ-3 (a, b) and CAZ-5 (c, d) alloys exposed to oxygen-saturated LBE at 550 oC for 10000 h
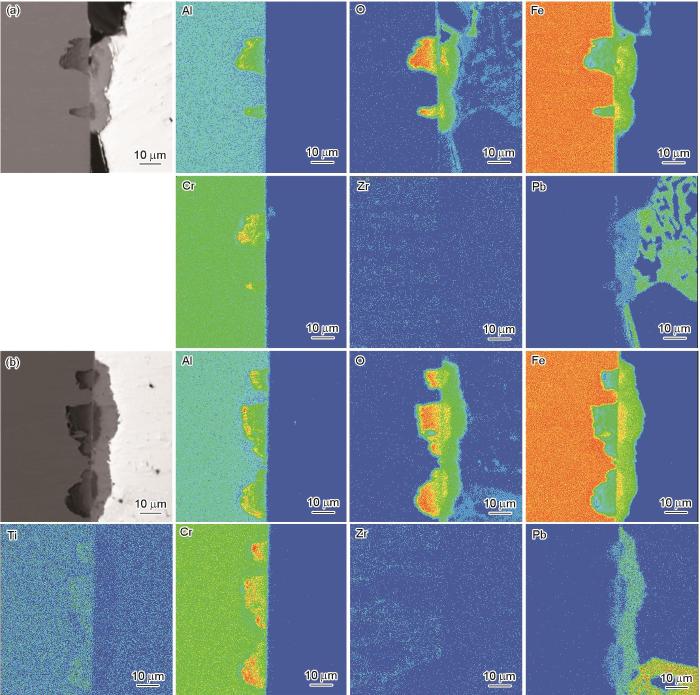
CAZ-5合金试样上多层氧化产物的EPMA元素面扫描结果如图9所示。与上述合金腐蚀产物一致,CAZ-5合金疖状氧化物的最外层为Fe3O4,内层主要为尖晶石结构FeCr2O4与Fe(Cr, Al)2O4氧化物。
图9
图9
经550 ℃、饱和氧LBE腐蚀10000 h后CAZ-5合金表面疖状腐蚀产物截面的EPMA元素面扫描结果
Fig.9
EPMA elemental mapping results of cross-section of oxide nodules on CAZ-5 alloy exposed to oxygen-saturated LBE at 550 oC for 10000 h
此外,借助EDS线扫描分析,得到CAZ-3和CAZ-5合金试样的Al2O3薄膜厚度分别是(1.48 ± 0.09)和(1.62 ± 0.10) μm。该薄膜厚度变化与Engkvist等[25]利用空气氧化得到的Al2O3薄膜变化趋势一致:Al含量越高,Al2O3薄膜厚度越小。
2.4 O含量对含Zr ODS FeCrAl合金氧化膜组成的影响
图10
图10
经550 ℃、饱和氧LBE腐蚀10000 h后CAZ-1合金氧化产物表面与截面形貌的SEM像,及疖状氧化物的SEM像和EDS面扫描结果
Fig.10
Surface (a) and cross-sectional (b) SEM images of oxide products, and SEM image and corresponding EDS mapping results of oxide nodules (c) on CAZ-1 alloy exposed to oxygen-saturated LBE at 550 oC for 10000 h
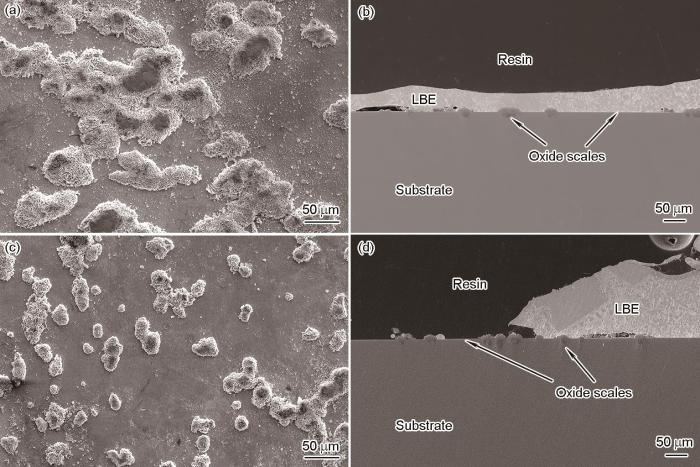
图11给出CAZ-4 (O含量0.10%)和CAZ-5 (O含量0.23%)合金经10000 h LBE腐蚀后表面形貌与截面形貌的SEM像。可见,腐蚀产物同样由Al2O3和疖状氧化物组成。EDS线扫描分析结果显示,CAZ-4和CAZ-5试样的Al2O3薄膜厚度分别为(1.59 ± 0.08)和(1.62 ± 0.10) μm,略大于Al含量较高的CAZ-1、CAZ-2和CAZ-3合金试样。而且,通过在不同视场下的统计可知,CAZ-4合金表面的疖状氧化物数量比CAZ-5合金多,再次证明ODS合金的O含量降低,不利于FeCrAl合金表面Al2O3膜的生成。
图11
图11
经550 ℃、饱和氧LBE腐蚀10000 h后CAZ-4和CAZ-5合金氧化产物表面与截面形貌的SEM像
Fig.11
Surface (a, c) and cross-sectional (b, d) SEM images of oxide products on CAZ-4 (a, b) and CAZ-5 (c, d) alloys exposed to oxygen-saturated LBE at 550 oC for 10000 h
3 分析与讨论
3.1 含Zr ODS FeCrAl合金的氧化行为
对于ODS合金,Ti、Zr、Al、Y等活性元素添加到合金中,会成为Y-Al-O、Y-Ti-O和Y-Zr-O等纳米强化相的组成元素,但是不会完全用于纳米氧化物的析出,部分会留存于合金基体中[27],影响合金的固溶强化、晶粒细化和耐腐蚀性能。表2的结果表明,含Zr ODS FeCrAl合金中Al、Ti和O含量的微量变化,导致基体内Y-Al-O、Y-Ti-O和Y-Zr-O相纳米颗粒的数量有很大的变化,进而对合金腐蚀行为有影响的元素浓度也发生变化,完全不同于表1所示的化学成分。然而腐蚀实验结果表明,即使是Y-Al-O相纳米颗粒生成最少、基体剩余Al含量最高的CAZ-2试样,合金表面也产生了疖状氧化物,该结果与前期报道的ODS FeCrAl合金(Fe-13.5Cr-4Al-0.3Ti-0.36Y2O3和Fe-11Cr-5Al-0.3Ti-0.38Y2O3)表面生成连续致密的Al2O3薄膜[10]明显不同。因为含Zr ODS FeCrAl合金与文献[10]的合金具有相同的制备工艺和腐蚀环境,由此推断该系列合金表面疖状氧化物的产生与合金添加的Zr元素密切相关。
早期的研究[28,29]认为,添加活性元素(reactive element,RE),如Y、Zr、La、Ce、Hf,对合金表面形成Al2O3薄膜有益。RE和RE的氧化物添加到合金中,不仅可以通过减小合金晶粒尺寸、增加位错和亚晶界密度使得大量Al到达表面,促进保护性Al2O3的快速形核与成膜[28],而且可以通过扩散,聚集到氧化膜的晶界上,阻挡金属离子向外扩散,使得Al2O3膜的生长速率下降[29]。对于ODS FeCrAl合金,基体内既弥散析出富Y的纳米氧化物,又具有高密度的晶界和位错,因此氧化初期有利于Al2O3薄膜的快速形成,使得保护性Al2O3薄膜生成的最低温度降到500 ℃[30,31]。然而,近年来的研究[32,33]表明,RE掺杂对抗氧化能力提升的有效性受到RE添加浓度及分布的影响,过量添加可产生负面作用。Smialek等[32]在对Fe-40Al-1Hf、Fe-40Al-1Hf-0.4B和Fe-40Al-0.1Zr-0.4B (原子分数,%)合金的等温氧化实验中,验证了其Al2O3膜的生长速率大约是NiAl-0.1Zr合金的5倍;同时,在200 cyc、1 h的循环氧化实验中,观察到了Fe-40Al-1Hf-0.4B和Fe-40Al-0.1Zr-0.4B合金表面氧化皮的起皱与大面积剥落,并将这些现象的产生归因于氧化产物中HfO2、ZrO2颗粒的形成及其与基体的热膨胀差异。Ejenstam等[34]对比研究了Zr、Y添加的FeCrAl合金(Fe-10Cr-4Al)在450~550 ℃铅熔液中的腐蚀行为,发现在Zr、Y含量较高的合金中均生成Fe3O4层。过量的Zr、Y元素会诱发脆性金属间化合物生成,例如Laves相,从而促进了Fe、Cr混合氧化物的产生。借助高温(≥ 1200 ℃)实验及FactSage热化学计算软件,Maeda等[26]也验证了较高浓度的Zr元素对空气和水蒸气氧化过程中ODS FeCrAl合金(Fe-15Cr-7Al-0.5Ti-0.4Zr-0.5Y2O3)的不利影响:过量Zr元素向氧化膜/合金界面处迁移,促使ZrO2颗粒在氧化膜内生成,然后,外界氧通过ZrO2颗粒向内扩散,氧化速率增加。
一般情况下,在升温过程中,FeCrAl基合金表面的Fe、Cr、Al等元素与O结合发生氧化反应,由于Al在氧化皮/合金界面高度富集,合金表面快速生成Al2O3膜,进入稳定氧化阶段后,最初生成的Fe和Cr的氧化物被Al2O3膜结合或溶解[29,35],后续Al2O3的主要变化为膜厚的增加。本工作含Zr ODS FeCrAl试样虽然在晶界(图2)的辅助下促进了Al2O3的形成,但是合金表面附近未参与Y-Zr-O相纳米颗粒析出而留在基体内的Zr元素,因其较大的离子半径和很强的亲氧性,可能会先于Al元素与O结合、并在晶界上抑制Al元素向表面扩散及其与O结合[29,36],使得该区域附近的Al2O3生成受阻,而此时Fe的氧化物形核。表面附近的Zr与O反应生成ZrO2微小颗粒后,可能与合金基体和氧化物之间的热膨胀系数不匹配[37],在应力作用下,ZrO2颗粒附近产生缺陷与微裂纹[38]。该缺陷和微裂纹与Fe元素向外扩散后在表面处形成的空位一起成为外界LBE与O向内扩散的通道,O向内传输并在微孔中生成新的氧化物,发生内氧化。由于Cr的扩散速率小于Fe,富Cr氧化物出现在内部氧化层。由于内部氧化层的阻挡,靠近基体的O浓度较低,此浓度只能达到Al、Cr氧化的浓度,因此IOZ主要由Al、Cr的氧化物组成。该区域疖状氧化物的产生类似于LBE环境下FeCr合金发生的“可用空间模型”机制[39~41]。上述推论可以很好地解释CAZ-2合金上疖状腐蚀产物数量最少的原因,因为CAZ-2合金中用于Y-Zr-O相析出的Zr元素最多(表2),而对腐蚀有影响Zr元素最少,从而有助于Al2O3的生成。图12给出了过量Zr元素对ODS FeCrAl合金氧化膜生成影响的示意图。
图12
图12
含Zr ODS FeCrAl合金腐蚀机制示意图
Fig.12
Schematics of corrosion mechanism of ODS FeCrAl alloy containing Zr
(a) alloying elements and O diffusing outward and inward at the initial stage of oxidation
(b) Zr preferentially binding to O concomitant with blocking Al and O binding
(c) Fe oxides nucleation assisted by Zr and O binding
(d) formation of Fe oxide nodules and continuance of internal oxidation
3.2 Ti、Al、O含量对含Zr ODS FeCrAl合金氧化行为的影响
上述表征和分析证明,(4.0%~5.5%)Al的添加不能阻止含Zr ODS FeCrAl合金表面生成Fe的疖状氧化物。利用多个2.5 mm × 1.8 mm的试样表面的SEM视场,统计疖状腐蚀产物的表面横向尺寸、表面个数;利用多个0.83 mm × 0.55 mm的试样截面视场,统计疖状氧化产物的平均厚度,结果如图13所示。结果表明,Ti、Al、O含量对ODS FeCrAl合金上疖状氧化物的分布和形貌也有影响。
图13
图13
5种合金疖状腐蚀产物的表面平均横向尺寸和平均厚度及数密度
Fig.13
Statistical results of average surface transverse dimension and average thickness (a), number density (b) for oxide nodules on the five alloys
3.2.1 Ti含量的影响
根据Heinzel等[42]报道,决定FeCrAl基合金Al2O3薄膜的形成有2个关键因素:一是合金中Al含量;二是合金元素的扩散速率。对于Al、Zr、O含量接近及加工工艺相同的CAZ-2和CAZ-3合金,虽然CAZ-2合金中Y-Al-O相纳米氧化物在所有析出相中的数量分数(4.1%)略微高于CAZ-3合金(2.6%),但是考虑到CAZ-2和CAZ-3合金析出相体积密度(3.89 × 1022和4.00 × 1022 m-3)相近,可以认为CAZ-2和CAZ-3合金中未参与强化相析出、而用于氧化行为的Al含量相近,因此,CAZ-3合金中疖状氧化物数量和氧化物厚度相较CAZ-2合金增加与活性元素Ti有关。Ti作为一种强亲氧元素,与Zr类似,也具有强吸氧效应[43]。氧化初期,局部合金表面的Ti、Zr元素协同,氧分压较低时与O结合,抑制了合金/氧化皮界面的氧分压增加及Al的氧化,进而导致高浓度的Fe与O反应,产生Fe的氧化物。同时,Ti4+离子半径(0.068 nm)与Fe3+、Cr3+离子半径(0.064和0.069 nm)相当,温度高于900 ℃时能表现出明显的向外扩散[44,45]。本工作的实验温度较低,随着氧化层由外向内O浓度的降低,Ti元素与Al一起发生内氧化[43,46,47],增加了内氧化向基体延伸的趋势。因此,CAZ-3合金的疖状氧化物数量和厚度相对无Ti的CAZ-2合金都增加。
3.2.2 Al含量的影响
此外,图13a的结果显示,CAZ-3合金疖状氧化物的表面横向尺寸相对较大。因为氧化产物优先在晶界处生成[48],根据腐蚀产物的截面形貌和合金试样的晶粒形貌,可以判定疖状氧化物的截面宽度是富Fe氧化物沿合金横截面晶粒宽度上发生的延伸。由图2可知,CAZ-3试样的晶粒宽度小于CAZ-5试样。CAZ-3试样上较多和较平直的晶界有利于Zr元素的向外扩散,增加了Fe的氧化物在合金表面的形核;同时,较小的晶粒宽度为Fe的氧化物与附近晶界氧化物的相连或结合提供了助力,更容易发生沿着合金晶粒宽度方向上氧化物的延伸,所以CAZ-3合金上大多数疖状氧化物的表面横向尺寸是合金基体多个晶粒宽度的组合(见图5c、6b和8a)。
3.2.3 O含量的影响
对于ODS合金来说,合金的O含量(或过量O含量)是决定纳米氧化物尺寸和组成的关键因素。对于未添加Zr元素的ODS FeCr和ODS FeCrAl合金,强化相分别是Y-Ti-O和Y-Al-O相纳米颗粒,O含量变化可以改变纳米强化相的组成和尺寸。少量Zr元素添加到ODS FeCrAl合金后,Y-Zr-O相纳米颗粒替代Y-Al-O相优先析出,结果如表2所示,O含量增加促进Y-Zr-O相纳米颗粒的析出。CAZ-2合金中Y-Zr-O相的析出数量明显大于CAZ-1合金,CAZ-5合金中Y-Zr-O相的析出数量大于CAZ-4合金,因此,CAZ-2和CAZ-5合金基体中对Al2O3膜生成不利的Zr元素含量相对降低,发生Fe的氧化及接下来内氧化的概率降低,在氧化行为上表现为随着O含量的增加(CAZ-1和CAZ-2合金对比,CAZ-4和CAZ-5合金对比),疖状氧化物的生成数量减小。另一方面,含Zr ODS FeCrAl合金的O含量降低,Y-Al-O相纳米氧化物的数量占比增大(表2),即使考虑了合金内纳米强化相的体积密度,CAZ-1和CAZ-4合金用于Y-Al-O相纳米颗粒析出的Al含量也依然较CAZ-2和CAZ-5合金多,因此用于Al2O3膜生成的Al含量降低。在较多Zr元素干扰和较低Al元素抵抗的共同作用下,Fe的氧化物更容易在CAZ-1和CAZ-4合金表面晶界处形核与生长,然后在单个或多个晶粒宽度上侧向延伸,使这2种合金表面疖状氧化物横向尺寸的范围较大。
4 结论
(1) 对含Zr ODS FeCrAl合金开展了550 ℃、10000 h、饱和氧LBE的浸泡腐蚀实验,发现合金表面同时存在Al2O3与Fe的疖状氧化物。少量Zr元素的添加促进了ODS FeCrAl合金表面Fe的疖状氧化物生成。
(2) 增加含Zr ODS FeCrAl合金的O含量,可以促进小尺寸的Y-Zr-O相纳米颗粒高密度析出,降低纳米氧化物的平均颗粒尺寸。
(3) 增加含Zr ODS FeCrAl合金的O含量,使得较多Zr元素用于纳米氧化物的析出,氧化初期干扰Al与O结合的Zr元素含量降低,进而降低含Zr ODS FeCrAl合金生成疖状氧化物的数量。
(4) 含Zr ODS FeCrAl合金中添加适量Ti元素,使得纳米尺寸的Y-Ti-O相与Y-Al-O和Y-Zr-O相纳米氧化物同时析出。在氧化初期与Zr元素一起阻碍Al与O结合,导致疖状氧化物的数量、厚度和表面横向尺寸都增加。
(5) Zr含量在0.3%左右时,4.0%~5.5%范围的Al含量不能阻止ODS FeCrAl合金表面上Fe的氧化产物的生成。若要消除含Zr ODS FeCrAl合金表面上的疖状腐蚀产物,获得连续、保护性的Al2O3薄膜,Al含量需要进一步提高。
参考文献
Corrosion behavior of 316L and T91 steels in stagnant lead-bismuth eutectic at 550 oC
[J].
316L和T91不锈钢在550 ℃静态铅铋合金中的腐蚀行为
[J].
Influence of temperature on the interaction mode of T91 and AISI 316L steels with Pb-Bi melt saturated by oxygen
[J].
On corrosion properties of ceramic materials for pump friction pairs in lead-bismuth environment
[J].
Compatibility of surface-coated steels, refractory metals and ceramics to high temperature lead-bismuth eutectic
[J].
Characterization of oxide dispersoids and mechanical properties of 14Cr-ODS FeCrAl alloys
[J].
Comparison of microstructure and microtexture evolution in 9Cr and 18Cr oxide dispersion-strengthened steels during fuel clad tube fabrication
[J].
Behavior of FeCrAl-ODS cladding tube under loss-of-coolant accident conditions
[J].
Corrosion behavior of Al-alloying high Cr-ODS steels in lead-bismuth eutectic
[J].
Effect of silicon and aluminum addition on corrosion behavior of ODS iron-based alloys in liquid lead-bismuth eutectic
[J].
Advanced TEM characterization of oxide nanoparticles in ODS Fe-12Cr-5Al alloys
[J].
Preliminary study on the fabrication of 14Cr-ODS FeCrAl alloy by powder forging
[J].A simple powder forging process was presented herein to fabricate an Fe-14Cr-4.5Al-2W-0.4Ti-0.5Y2O3 ODS FeCrAl alloy. The forged alloy exhibits a high density that exceeds 97 % of the theoretical density. The ODS alloy was investigated in terms of the residual porosity, morphology and phase structure of oxide nanoparticles, impact toughness and tensile properties. It was found that refined grains were obtained during powder forging. A residual porosity less than 1.1 % has no impact on the precipitation of oxide nanoparticles. The average diameter of the oxide particles is 7.99 nm, with a number density of 2.75 × 1022 m-3. Almost all of the oxides are identified as orthorhombic YAlO3 particles. The refined grains and uniformly distributed oxide nanoparticles enable the alloy to show excellent mechanical strength and ductility below 700 °C, and enable the ductile-to-brittle transition temperature to be close to room temperature. However, a slight decrease in strength at 1000 °C and the Charpy upper shelf energy has been suggested to be due to the residual porosity. These results indicate that powder forging can be used as a promising technique for the fabrication of ODS alloys.
Ultra-high temperature tensile properties of ODS steel claddings under severe accident conditions
[J].
Effect of pre-oxidation treatment on the corrosion resistance in stagnant liquid Pb-Bi eutectic of 12Cr ferritic/martensitic steel
[J].Lead-cooled fast reactors using liquid lead or lead-bismuth eutectic (LBE) alloy coolants have attracted international attention due to their unique advantages in safety, economy, and sustainable development. The availability of suitable core materials is one of the key challenges restricting the development and application of the lead-cooled fast reactor technology. Ferritic/martensitic steel is one of the important candidates for nuclear reactor fuel cladding, but the dissolution of Cr and Ni occurs in it upon contact with high-temperature LBE, resulting in cladding failure. Adding Si can improve corrosion resistance, and based on this property, previous work developed a high-Si ferritic/martensitic steel for LBE alloy-cooled fast reactor. Recently, pre-oxidation treatment was proposed to further improve the corrosion performance of steel in contact with LBE. However, the structure of the oxide film formed after the pre-oxidation of 12Cr ferritic/martensitic steel, the effect on corrosion resistance, and the failure mechanism are not clear. In this study, a pre-oxidized film was formed on the steel surface and its structure was characterized. Steel corrosion experiments using oxygen-saturated LBE at 550oC were also performed to analyze the influence of pre-oxidation treatment on the LBE alloy coolant corrosion resistance of steel. The results demonstrated that the oxide films formed when steel is pre-oxidized at 720oC in 1%O2 + 99%N2 atmosphere for 1 h are mainly (Fe, Cr)2O3 and MnCr2O4 oxides. The oxide films can effectively prevent the outward diffusion of Fe in steel and the inward diffusion of O in LBE, thereby improving the corrosion resistance of steel to stagnant oxygen-saturated LBE alloy coolant at 550oC. However, due to the high diffusion rate of Mn and its high solubility in LBE alloys, the Mn in the pre-oxidized film will gradually diffuse and dissolve into the LBE alloy, rendering subsequently a part of the oxide film ineffective and forming a localized corrosion zone. After 1000 h of Pb-Bi corrosion, the local corrosion area on the alloy surface can reach 60%. This study revealed the microstructure, protective effect, and failure mechanism of the pre-oxidized film on the surface of high-Si ferritic/martensitic steel, and suggested research directions for further improving the effectiveness and stability of the pre-oxidized film.
预氧化处理对12Cr铁素体/马氏体钢耐Pb-Bi腐蚀性能的影响
[J].
Effects of Zr content on the microstructure of FeCrAl ODS steels
[J].
Effect of Zr content on microstructure and hardness of ODS-FeCrAl alloys
[J].
Effects of contents of Al, Zr and Ti on oxide particles in Fe-15Cr-2W-0.35Y2O3 ODS steels
[J].
Effect of Zr and Al addition on nanocluster formation in oxide dispersion strengthened steel—An ab initio study
[J].
Advanced FeCrAl ODS steels for high-temperature structural applications in energy generation systems
[J].
Corrosion behaviors of FeCrAl alloys exposed to oxygen-saturated static lead bismuth eutectic at 550 oC
[J].
Temperature effect on the corrosion mechanism of austenitic and martensitic steels in lead-bismuth
[J].
Effect of silicon on the oxidation resistance of 9 wt.% Cr heat resistance steels in 550 oC lead-bismuth eutectic
[J].
Research advance on liquid lead-bismuth eutectic corrosion resistant Si enhanced ferritic/martensitic and austenitic stainless steels
[J].Structural materials are one of the major factors that restrict the lead-cooled fast reactor construction due to metallic elements that can dissolve in the liquid lead-bismuth eutectic (LBE), which may affect the structure's safety. T91 steel and 316 stainless steel are the leading structural materials for critical equipment such as fuel cladding, reactor vessels, and reactor core internals. The environmental compatibility of those steels with the liquid LBE needs to be systematically evaluated. However, T91 steel and 316 stainless steel suffer from rapid oxidation corrosion in oxygen-saturated LBE at 550oC. T91 steel's corrosion resistance in liquid LBE can be improved by decreasing the oxygen concentration (1.26 × 10-6%, mass fraction), but dissolved corrosion occurred at dissolved oxygen concentration below 1 × 10-6% for T91 steel and 316 stainless steel. T91 steel is sensitive to liquid metal embrittlement, significantly reducing its corrosion fatigue life in the liquid LBE. Compared to the standard (9%-12%)Cr ferritic/martensitic steel and 316 stainless steel, the microalloyed Si enhanced (9%-12%)Cr ferritic/martensitic steel (9Cr-Si and 12Cr-Si) and 316 stainless steel (ASS-Si) have good microstructural stability and comprehensive mechanical properties. The Si-rich oxide formation in liquid LBE improves the oxide film compactness and corrosion resistance. The dissolution corrosion was inhibited in static oxygen-saturation and oxygen-controlled (10-6%-10-7%) flowing liquid LBE (0.3 m/s) at 550oC for 9Cr-Si, 12Cr-Si, and ASS-Si. These alloys are expected to meet the design requirements for a lead-cooled fast reactor.
耐Pb-Bi腐蚀Si增强型铁素体/马氏体钢和奥氏体不锈钢的研究进展
[J].结构材料是制约铅冷快堆建设的关键因素之一,原因是其组成元素在液态Pb-Bi共晶(LBE)中会发生不同程度的溶解,影响结构安全。候选结构材料铁素体/马氏体钢T91与不锈钢316在550℃饱和氧LBE环境中发生快速氧化腐蚀;溶解氧浓度降至1.26 × 10<sup>-6</sup>% (质量分数)可减轻T91的液态LBE腐蚀,但低于1 × 10<sup>-6</sup>%时,T91与316钢发生溶解腐蚀;T91液态LBE脆化敏感性高,导致其在350℃液态LBE中腐蚀疲劳寿命显著降低。与商用的(9%~12%)Cr铁素体/马氏体钢和316型奥氏体不锈钢相比,经微合金化的Si增强型铁素体/马氏体钢(9Cr-Si和12Cr-Si)和奥氏体不锈钢(ASS-Si),具有较好的组织稳定性和综合力学性能,且在液态LBE中形成的富Si氧化物提高了氧化膜的致密性,改善了其耐腐蚀性能,在550℃下静态饱和氧和动态控氧LBE环境中的溶解腐蚀受到抑制,有望满足铅冷快堆的设计需求。
Oxide scales formed on Fe-Cr-Al-based model alloys exposed to oxygen containing molten lead
[J].
High temperature oxidation of FeCrAl-alloys—Influence of Al-concentration on oxide layer characteristics
[J].
Effects of zirconium and oxygen on the oxidation of FeCrAl-ODS alloys under air and steam conditions up to 1500 oC
[J].Effects of Zr and excessive oxygen addition on the oxidation behavior of FeCrAl-ODS alloys are evaluated by high-temperature air and steam oxidation tests together with analyses using the thermochemical multiphase computer software FactSage. Zr addition to improve the strength of FeCrAl-ODS alloys increases the oxidation rate, but the oxidation rate can be reduced by increasing the excessive oxygen content to the appropriate level. From thermochemical multiphase calculations, Zr activity becomes lower in the alloy matrix with increasing excessive oxygen, and preventing ZrO2 formation within the alumina scale can decelerate oxygen inward diffusion through ZrO2. Addition of Zr and excessive oxygen can also improve adhesion of the alumina scale at 1400 degrees C. Once the protective alumina scale is lost during oxidation at 1500 degrees C, Fe-O direct reaction with exothermic heat takes place and an oxidation reaction with partial melting rapidly proceeds within 30 min. (C) 2019 Elsevier B.V.
Alloy design and characterization of a recrystallized FeCrAl-ODS cladding for accident-tolerant BWR fuels: An overview of research activity in Japan
[J].
Effects of Dy on cyclic oxidation resistance of NiAl alloy
[J].
Corrosion of ODS steels in lead-bismuth eutectic
[J].
Influence of alloy composition and temperature on corrosion behavior of ODS ferritic steels
[J].
Oxidation behavior of FeAl + Hf, Zr, B
[J].
Optimization of reactive-element additions to improve oxidation performance of alumina-forming alloys
[J].
Optimizing the oxidation properties of FeCrAl alloys at low temperatures
[J].
Composition and growth mechanisms of alumina scales on FeCrAl-based alloys determined by SNMS
[J].
Current thoughts on reactive element effects in alumina-forming systems: In memory of John Stringer
[J].
Review: Mechanism of reactive element effect—Oxide pegging
[J].High temperature protective coatings are involved in a wide variety of applications including aero engines and gas turbines. Reactive elements, including all rare-earth elements as well as Ti, Zr, and Hf, are increasingly used to modify high temperature protective coatings, and their main effects are reducing scale growth and improving scale adhesion. The mechanism through which reactive elements work is yet to be clearly understood. The current mechanism comprises “enhanced scale plasticity”, “graded seal mechanism”, “modification to growth process”, “chemical bonding”, “the vacancy sink model”, “oxide pegging”, “dynamic segregation theory”, and “the sulfur effect theory”. Among these, oxide pegging is perhaps the most important one, although some people may disagree. Oxide pegging is the result of the mechanical joining of an oxide to its corresponding alloy; it is a result of either the internal oxidation of added reactive elements or dispersed oxide particles growing in size and extending into the alloy. This paper offers an overview of the research progress on oxide pegging, including its proposed, the relationship between the peg and scale adherence, improving the “key-on” effect, and the peg formation and growth under different conditions (the doped reactive element with a low or high solid solute in the alloy, dispersed oxide added in the alloy, and two reactive elements doped into the alloy). Moreover, it sheds light on the model’s inability to explain the surface application of reactive elements on the alloy. Finally, the paper suggests future studies on this model, like focusing on how to obtain the ideal oxide pegging, developing a new model for oxide peg formation and growth with two or more reactive elements added to the alloy, and the cooperation effect between the oxide pegging and other mechanisms. The paper’s objective is to offer a better understanding of oxide pegging and to provide theoretical support for the studies on the reactive element effect and the design of materials in thermal barrier coating systems.
综述: 活性元素作用机理——氧化物“钉扎”模型
[J].
Microstructure of oxide film and nodular corrosion mechanism of zircaloy-4 alloy
[J].
Zr-4合金氧化膜显微组织与疖状腐蚀机制研究
[J].
Oxidation mechanism of a Fe-9Cr-1Mo steel by liquid Pb-Bi eutectic alloy (Part I)
[J].
Oxidation mechanism of an Fe-9Cr-1Mo steel by liquid Pb-Bi eutectic alloy at 470 oC (Part II)
[J].
Oxidation mechanism of a Fe-9Cr-1Mo steel by liquid Pb-Bi eutectic alloy (Part III)
[J].
Corrosion of steels with surface treatment and Al-alloying by GESA exposed in lead-bismuth
[J].
Effect of titanium on oxidation behavior of high-purity ferritic stainless steel
[J].
High temperature oxidation of Fe-18Cr alloys with small amounts of Ti
[J].
Effect of titanium addition on the oxidation resistance of Fe-13Cr-5Al-0.3Ti alloy in air between 700 oC-1100 oC
[J].
Transmission electron microscopy (TEM) study of the oxide layers formed on Fe-12Cr-4Al ferritic alloy in an oxygenated Pb-Bi environment at 800 oC
[J].
Influence of Ti addition on oxidation behavior of Ni-Cr-W-based superalloys
[J].