金属基植入材料(如不锈钢、钛基合金和钴基合金)因其优异的力学性能,已广泛应用于髋关节和膝关节假体以及骨折固定针、钉和板等承重部位[5,6]。相比于永久性金属基植入材料,可降解医用金属在骨组织愈合后,能够在生物体内分解代谢,并展现出良好的骨愈合率,克服了不锈钢、钛基合金和钴基合金等需二次手术取出的局限性[7]。在所有金属基植入材料中,可降解医用Mg及镁合金具有与皮质骨最接近的弹性模量和密度[8,9]。1900年,Payr[10]首次提出在关节置换术中使用镁板来恢复关节运动,极大推动了Mg在骨科领域的应用。不同于永久性金属植入材料溶出的金属离子会在体内积累,Mg及镁合金具有生物可降解特性,其腐蚀产物具有生物安全性,且腐蚀生成的Mg为人体必需元素(成人建议Mg摄入量为240~420 mg/d[11])。
目前,全球已有少量镁合金及其复合材料应用于骨科植入器械并实现临床转化的成功案例。然而,多数医用镁基复合材料的研究仍处于实验室或临床前阶段。2013年,德国Syntellix公司生产的MAGNEZIX镁合金螺钉(Mg-Y-RE-Zr合金)成为首个获得欧盟CE认证的镁基骨科植入体[12]。2015年,韩国U&I公司生产的RESOMET/K-MET镁螺钉(Mg-Ca合金)也通过韩国食品药品管理局批准,主要用于手部骨折内固定手术[13]。临床研究[14]显示,MAGNEZIX镁合金螺钉在骨折治疗中效果显著,在一项针对29名骨折患者的实验中,所有患者均恢复良好,无炎症反应。Jungesblut等[15]进一步采用MAGNEZIX镁合金螺钉治疗不稳定型剥脱性骨软骨炎病变及移位的骨软骨碎片的固定。经过对19例患者为期11个月的观察,12例患者完全愈合,1例因膝关节内镁钉折断移位需手术修复,其余患者术后均未出现炎症或新的脱位。上述结果表明,MAGNEZIX镁合金螺钉为骨折愈合提供了有效支持。
国内对镁基植入体的审批和注册政策逐步完善,重点聚焦于植入体的耐腐蚀性能、可控降解性以及体内降解风险评估标准等关键问题。2020年,东莞市宜安科技有限公司研发的国内首例高纯镁骨钉获得欧盟CE认证[16]。上海交通大学研发的Mg-Nd-Zn-Zr (JDBM)镁合金骨板及螺钉在骨科临床实验取得突破性进展[17]。此外,中国科学院深圳先进技术研究院以聚乳酸-羟基乙酸共聚物(poly(lactide-co-glycolide),PLGA)、β-磷酸三钙(β-tricalcium phosphate,β-TCP)和Mg粉为原料,成功制备了用于骨修复的3D打印PLGA/TCP/Mg复合支架。该产品已通过国家药品监督管理局创新医疗器械特别审批,并完成了临床前注册检测,目前正处于多中心临床实验阶段[18]。
尽管传统金属基骨科植入物已广泛应用于临床手术,但其在应对不同个体的骨科疾病时逐渐显现出局限性,难以满足多样化的治疗需求。为了应对这些挑战,近年来大量研究致力于开发新型镁基复合材料,重点探索如何有效平衡其力学性能和降解速率。本工作重点讨论不同制备方法对镁基复合材料力学性能及耐腐蚀性能的提升作用。此外,概述了具有不同增强体的镁基复合材料的力学性能与体外降解行为。最后,总结了镁基复合材料在骨科植入器械领域的应用及相应的修复机制。
1 生物可降解镁基复合材料的制备方法
医用镁基复合材料的常用制备工艺可分为传统制备方法和新型制备方法。传统制备方法包括粉末冶金法、搅拌铸造法;新型制备方法包括分离熔体沉积法和增材制造法等[23]。
1.1 粉末冶金法
粉末冶金法作为一种制备镁基复合材料的固态烧结技术,因其操作简便、经济高效而得以广泛应用。其主要优点包括:增强体颗粒在基体中分布均匀、增强体与基体的比例可调控、制备温度与铸造法相比更低[24]。粉末冶金法包含以下3个基本步骤:粉末制备与混合、生坯成型和烧结。生坯通常在室温下制备,烧结在可控气氛中进行,防止氧化。此外,为了满足不同材料体系的性能要求,发展了一些先进的烧结技术,如热压烧结、微波烧结和放电等离子烧结等,这些技术通过同时施加压力和温度,有效提高复合材料的致密度和均匀性。值得一提的是,粉末冶金法可以与后续的塑性加工技术(如热等静压、热挤压、轧制等)相结合,大幅提升复合材料的致密度、强度与韧性,从而满足高性能应用需求。
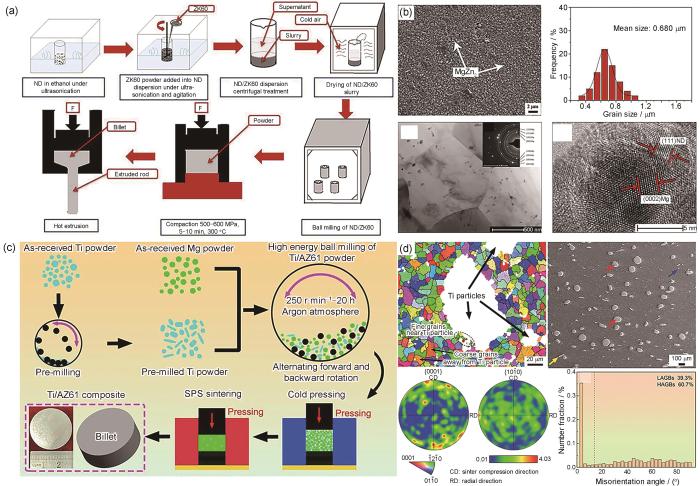
图1a[25]为粉末冶金法结合热挤压制备纳米金刚石(ND)增强ZK60镁合金复合材料制备流程示意图。为实现纳米级ND (约6.7 nm)与ZK60合金粉末的均匀混合,首先通过超声技术将ND分散于酒精溶液中,再加入ZK60合金粉末制备均匀悬浮液,经干燥和球磨处理后得到混合粉末。随后,通过热压烧结成预制件,并经热挤压制备出ND/ZK60复合材料。从图1b[25]可以看出,ND颗粒均匀分布在Mg基体中,无明显界面反应,显著阻碍了晶粒生长,使Mg基体的晶粒细化至约0.68 µm。此外,添加0.05%ND (质量分数,下同)的复合材料,其拉伸和压缩屈服强度分别提升至390和430 MPa,显著优于ZK60合金。Gu等[26]采用粉末冶金法成功制备了羟基磷灰石(HA)增强镁基复合材料。当微米级HA含量为10%时,HA能够均匀分布在Mg基体中,此时复合材料的屈服强度最高。随着HA含量进一步提高,HA在Mg基体中出现团聚现象,HA/Mg复合材料强度和韧性均有所下降。Munir等[27]通过粉末冶金法开发了石墨烯纳米片(GNPs)增强镁基复合材料,通过高能球磨工艺将低含量的GNPs (0.1%、0.2%和0.3%)均匀分布在Mg基体中,在避免GNPs团聚的同时,保持了GNPs的结构完整性,提高了GNPs/Mg复合材料的抗压强度、延展性和耐腐蚀性能,体外细胞测试表明GNPs/Mg复合材料均具有良好的生物相容性。
图1
图1
粉末冶金法制备镁基复合材料的流程示意图及显微组织[25,29]
Fig.1
Schematic of the preparation process of nano-diamond (ND) reinforced ZK60 composite via powder metallurgy[25] (F—force) (a) and microstructures[25,29] (b-d)
(b) SEM image of ND/ZK60 composite, corresponding grain size distribution, and TEM and high-resolution TEM (HRTEM) images at the interface[25] (Inset in Fig.1b is selected area electron diffraction (SAED) pattern in the corresponding region)
(c) schematic of the preparation process of Ti/AZ61 composite prepared by spark plasma sintering (SPS)[29]
(d) electron backscatter diffraction (EBSD) and SEM images and kernel average misorientation (KAM) results of Ti/AZ61 composite (LAGBs—low angle grain boundaries, HAGBs—high angle grain boundaries)[29]
粉末冶金法特别是机械合金化,已被证明是制备微纳米金属颗粒增强Mg基体的可行方法。尤其是针对Ti颗粒增强镁基复合材料,机械合金化有效地提高了Ti在Mg中的固溶度,促进了晶粒细化,并提高了Ti颗粒在Mg基体中的均匀分散性。Yu等[28]通过高能球磨细化了Mg基体和增强体,再结合真空热压与后续热挤压,成功制备了具有亚微米级Ti增强体弥散分布的Ti/AZ31复合材料。研究表明,随着球磨时间的延长,Mg基体的晶粒尺寸逐渐减小。当球磨时间达到110 h,Mg基体的平均晶粒尺寸约为66 nm,Ti增强体平均粒径约为340 nm,形成了纳米晶组织。该复合材料屈服强度高达341 MPa,相比于AZ31镁合金提高了2.4倍。
放电等离子烧结(SPS)是一种先进的粉末冶金工艺,利用高脉冲直流电通过Joule热效应加热粉末,需要的烧结温度更低,烧结时间更短,能够实现一步制备具有细小晶粒的致密件。Xu等[29]采用SPS制备了Ti颗粒增强AZ61镁基复合材料(图1c[29])。Ti/AZ61复合材料具有优异的综合力学性能,抗压强度和压缩塑性分别为502 MPa和13%。从图1d[29]可以看出,复合材料中多数Ti颗粒呈现粗的椭球形和较细的条带状形貌,且Ti颗粒周围分布着细小的Mg晶粒。这是因为在SPS致密化过程中,Mg基体不仅承受了较高的外部施加载荷,还承受了相对较硬的Ti颗粒施加的应力,导致Mg基体经历较大的塑性应变,进而促进了Mg基体动态再结晶晶粒成核,在Ti颗粒周围形成了细晶粒Mg区。
作为较早应用于医用镁基复合材料制备的方法,粉末冶金法能够有效分散增强体颗粒,促进增强体与基体之间的原子扩散,从而优化基体组织并提高复合材料的致密度。该技术的优势在于可以同时使用多种类型及不同体积分数的增强体颗粒,特别适用于制备小型、含复杂合金元素的Mg基体材料以及增强体含量较高的复合材料[23]。然而,由于镁基粉末具有易燃、易爆且在空气中易氧化的特性,如何安全制备作为原料的镁基粉末,仍是限制粉末冶金法应用于镁基复合材料制备的主要挑战。
1.2 搅拌铸造法
搅拌铸造是一种常见且经济的批量制备复合材料的方法,通过将熔体和增强体颗粒同时搅拌,从而实现增强体颗粒在基体中的均匀分散[30]。Radha和Sreekanth[31]通过搅拌铸造和热挤压结合的方法制备了纳米HA增强镁基复合材料,发现与纯Mg相比,Mg-Sn合金对纳米HA颗粒的润湿性更好,在搅拌铸造过程中能形成较高的界面结合强度,具有更高的抗压强度。然而,由于纳米颗粒间Van der Waals力很强,与Mg基体的润湿性很差,很难将纳米颗粒均匀地分散在Mg基体中。此外,为获得均匀的颗粒分布,需要较长的搅拌时间,这通常会导致Mg基体中产生过多的气体和氧化,且增强体颗粒与Mg基体的界面反应难以控制。
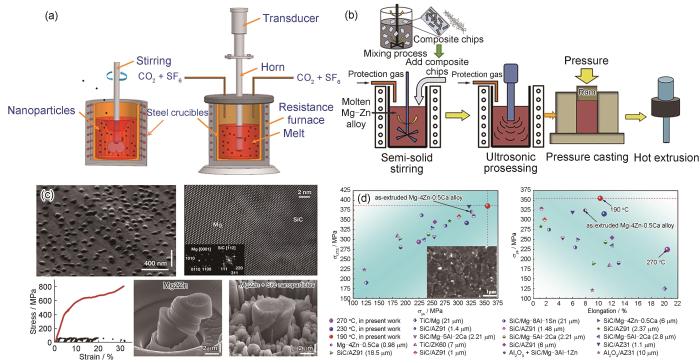
图2
图2
超声辅助搅拌铸造法制备镁基复合材料实验装置示意图和流程及显微组织和力学性能[33,37,39,40]
Fig.2
Schematics of the ultrasonic-assisted stir casting process[33] (a) and ultrasonic-assisted semi-solid stirring method for preparing graphene-reinforced Mg-based composites[37] (b) and microstructures and mechanical properties[39,40] (c, d)
(c) SEM image of nano-SiC reinforced Mg-Zn-based composites; Fourier-filtered atomic-resolution TEM image of SiC/Mg interface; compression engineering stress-strain curve and in situ SEM images of micro-pillar tests (Insets in Fig.2c are the fast Fourier transforms (FFT) of the magnesium matrix (left) and the SiC nanoparticle (right), respectively)[39]
(d) comparisons of mechanical properties of Mg-based composites (Inset in Fig.2d is SEM image of extruded nano-TiC reinforced Mg-Zn-Ca composites; σUTS—ultimate tensile strength, σys—yield strength)[40]
相比于传统搅拌铸造法,半固态搅拌法具有成型温度低、模具寿命长、晶粒细化、孔隙率低、缩孔少、铸件质量良好等优点[34]。Chen等[35]通过水流模拟实验,系统研究了半固态搅拌法中的关键工艺参数,包含搅拌速率、搅拌器高度和搅拌器与液体平面的夹角,确立了最佳参数组合(搅拌速率为100~950 r/min,高度为4~10 cm,夹角为75°)。随后,采用半固体搅拌与后续热挤压相结合的方法,成功制备了综合力学性能良好的Ti/AZ81复合材料,其拉伸屈服强度、抗拉强度和伸长率分别达到263 MPa、374 MPa和11.5%。与粉末冶金法相比,尽管半固态搅拌铸造制备的材料力学强度略有下降,但该方法简单、高效,显著降低了制备成本,在镁基复合材料大规模生产中展现出巨大的应用潜力。然而,半固态成型技术在实现纳米级增强体颗粒均匀分散方面依然面临挑战。这主要是由于纳米级增强体颗粒具有较大的比表面积,且在镁熔体中润湿性较差,易发生团聚现象,从而限制了其在镁基复合材料制备中的实际应用[36]。
超声振动在镁合金熔体中分散纳米增强体颗粒方面已显示出巨大的应用潜力,因而已有研究将超声波振动应用于辅助半固态成型工艺以实现微/纳米级增强体颗粒的宏观和微观分散。Xiang等[37]开发了一种预分散增强体颗粒工艺与超声辅助半固体搅拌铸造法相结合的制备工艺,实现了石墨烯在Mg基体中的良好分散,如图2b[37]所示。通过超声波将石墨烯分散于乙醇中,加入聚乙烯醇(PVA)及Mg屑,磁力搅拌后烘干,获得吸附石墨烯的Mg屑。随后将其加入半固态镁熔体中并利用超声波辅助分散,制备石墨烯增强镁基材料。最后,结合热挤压工艺,可进一步制备出具有优良强度和塑性的石墨烯增强镁基复合材料。Wang等[38]采用超声辅助半固体搅拌铸造法制备了微米级SiC增强镁基复合材料,并系统研究了半固态搅拌时间、超声处理时间对复合材料显微组织和力学性能的影响。结果表明,超声处理可显著缩短前期半固体搅拌时间,仅需5 min即可实现微米级SiC颗粒的预分散。随着超声波处理时间的增加,超声波的“热”效应增强,空化效应减弱。结合20 min的超声波处理,能有效改善SiC与Mg基体之间的润湿性,从而获得界面结合良好的复合材料。Chen等[39]发展了超声辅助搅拌铸造和蒸发法相结合的技术,成功获得了均匀分散的SiC纳米颗粒增强镁基复合材料。首先,采用超声辅助搅拌铸造工艺制备了含有1%SiC (体积分数)的Mg-6Zn复合熔体;然后,在真空炉中对复合熔体中的Mg基体进行蒸发处理,缓慢冷却后成功制备了具有14%SiC (体积分数)纳米颗粒增强Mg-2Zn镁基复合材料。如图2c[39]所示,纳米SiC颗粒与Mg基体界面属于半共格键合,微柱压缩实验结果表明,与Mg-2Zn样品相比,复合材料的屈服强度可高达410 MPa,且能够平稳地承受逐渐增加的载荷直至塑性应变超过30%。均匀分布的纳米SiC颗粒不仅可以大幅度提高镁基材料的力学强度,还可以使变形更加均匀和稳定。Nie等[40]进一步采用超声辅助半固体搅拌铸造法结合后续挤压工艺制备了TiC纳米颗粒增强Mg-4Zn-0.5Ca镁基复合材料。超声辅助搅拌实现了TiC纳米颗粒在Mg基体中的均匀分布,并且细化了Mg基体的晶粒尺寸,TiC纳米颗粒引起的粒子刺激形核(PSN)为挤压时的动态再结晶提供了巨大的驱动力,提高了复合材料的再结晶晶粒体积分数。复合材料的拉伸屈服强度可达355 MPa,同时延伸率为10.2%,均高于已报道的镁基复合材料力学性能,如图2d[40]所示。
因此,持续改进搅拌铸造工艺有望推动医用镁基复合材料部件的大批量、低成本、近净成型的工业化生产。特别是结合挤压、轧制和高压扭转等后续塑性变形工艺,可进一步细化晶粒结构,有望制备力学性能优异的镁基复合材料并可应用于骨科领域。
1.3 分离熔体沉积法
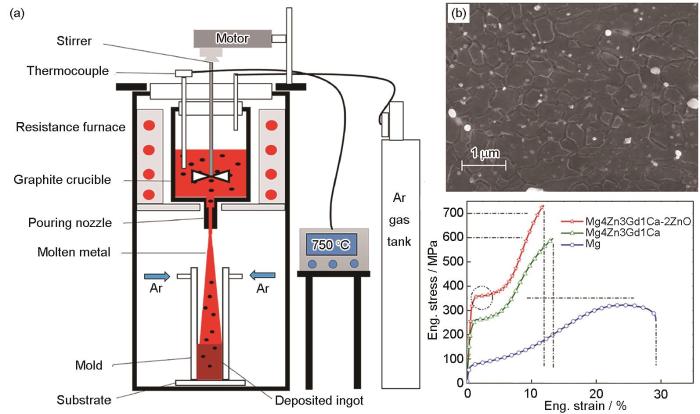
图3
然而,DMD制备得到的复合材料孔隙率大,必须结合后续塑性加工(通常采用热挤压)来消除气孔,且该方法设备较为复杂,难以制备大尺寸镁基复合材料。Xiang等[43]通过结合DMD技术与热挤压工艺,制备了低含量GNPs增强镁基复合材料(< 0.25%)。研究表明,DMD技术能够均匀分散GNPs,并显著提升复合材料的力学强度和延展性。这主要归因于GNPs促进了Mg基体的动态再结晶,并激活了非基面滑移与拉伸孪晶重新取向。Chen等[44]采用DMD与后续热挤压工艺制备了纳米ZnO增强Mg-4Zn-3Gd-1Ca复合材料。结果表明,纳米ZnO有效细化了Mg基体的晶粒,复合材料表现出优良的压缩强度和塑性,抗压强度高达703 MPa,压缩塑性为10.6%,与单纯的镁合金相比,抗压强度提高了约100 MPa (图3b[44])。Pahaul等[45]通过结合DMD技术与热挤压工艺,成功制备了纳米SiO2增强Mg-5Se-2Zn复合材料。与纯Mg相比,该复合材料压缩性能显著提升,其屈服强度提高了157%、抗压强度提高了54%、平均断裂强度提高了39%,能量吸收能力增加了112%。尽管复合材料的耐腐蚀性能低于纯Mg,但浸泡在体外磷酸盐缓冲液(PBS)中28 d后,复合材料仍保持了可接受的腐蚀速率和结构完整性,显示出在生物医学领域的良好应用潜力。
DMD技术用于大规模制备颗粒分布均匀、无团聚的小型高性能镁基复合材料产品。然而,增强体颗粒与基体的润湿性以及界面结合问题是开发高性能镁基复合材料的关键,仍需进一步研究。未来,DMD技术的主要挑战在于如何引入高体积分数的增强体(特别是纳米级增强体体积分数> 3%),因为熔体黏度的增加使得增强体的搅拌和均匀分布变得更加困难。
1.4 增材制造法
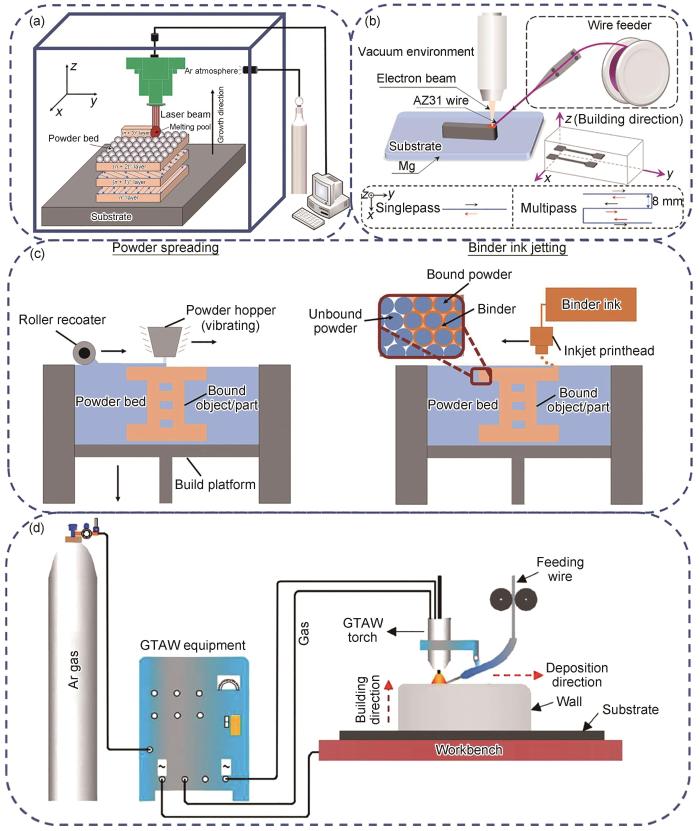
增材制造技术具有设计灵活、生产周期短以及沉积速率和效率高等优点。金属增材制造技术可分为粉床熔融和定向能量沉积[46]。增材制造过程中使用的热源可分为激光、电子束和电弧。根据热源的利用方式,金属增材制造技术可分为激光粉床熔融技术、电子束熔融技术、黏合剂喷射增材制造(BJAM)技术和电弧增材制造(WAAM)技术[47]。选区激光熔化(SLM)技术通过激光沿预定扫描路径在粉末床上进行加工,其装置示意图如图4a[48]所示。SLM技术具备极快的冷却速率,有助于晶粒细化。然而,需要注意的是,该工艺也可能导致样品中产生孔隙和微裂纹。由于镁合金粉末具有较高的蒸气压,采用SLM技术制备镁基复合材料仍面临诸多难题,因此关于该领域的研究相对较少。相比之下,电子束熔丝增材制造(EBAM)技术利用高能电子束作为热源,在真空条件下逐层熔化丝材,从而制备出致密度更高的样品,其装置示意图如图4b[49]所示。BJAM技术采用粉床工艺,通过喷墨打印头逐层喷射黏结剂选择性沉积在粉床上,黏结打印预制坯,随后对预制坯进行脱脂和烧结,最终获得成品,如图4c[50]所示。WAAM技术在制造零部件时,金属丝以恒定的速率送入,并在电弧作用下熔化到基板或之前沉积层上,如图4d[51]所示。与激光或电子束熔融增材制造技术相比,WAAM具有更高的沉积速率、更好的材料利用率以及节能效果,在制备大型镁基复合材料方面表现出巨大的潜力[52]。目前,关于WAAM的研究主要集中于通过改变工艺参数从而改善镁合金的各向异性,而关于应用WAAM技术制备镁基复合材料的相关研究还很少。
图4
相较于传统粉末冶金法和铸造法,采用SLM技术制备的镁基产品通常表现出较低的相对致密度,从而导致力学性能略有下降,这主要归因于镁合金熔点和沸点之间较小的温差以及较低的激光能量吸收率[53,54]。为了改善镁基产品的致密度,Wu和Wang[55]引入了比表面积大且激光吸收率高的碳纳米管(CNTs),并通过SLM工艺成功制备了CNTs/AZ31B复合材料。研究表明,添加1.5%CNTs后,CNTs/AZ31B复合材料的激光吸收效率从76.4% (AZ31B合金)提高到84.3%。此外,当能量密度提高至42 J/mm3时,镁基复合材料的熔池尺寸增大,复合材料的相对致密度达到最优水平,同时力学性能也显著提升,屈服强度达245 MPa,拉伸强度为286 MPa。Shuai等[56]利用氧化石墨烯(GO)对腐蚀介质的优异阻隔性能,采用SLM技术制备了GO增强AZ61镁基复合材料。通过超声分散分别处理GO和AZ61粉末,利用超声辅助搅拌使GO均匀附着于AZ61粉末。经真空蒸馏制备不同GO含量的混合粉末,最终通过SLM工艺制备GO增强AZ61镁基复合材料。结果表明,GO/AZ61复合材料具有三维蜂窝状纳米结构,其中蜂窝纳米结构由第二相GO颗粒构成,Mg晶粒被包裹在蜂窝状单元中。此外,GO有效细化了Mg基体的晶粒尺寸(从20.45 μm降低至8.38 μm),并显著提升了复合材料的耐腐蚀性能。这是因为GO蜂窝状纳米结构可作为一道紧密屏障,阻止腐蚀的扩展,同时GO上含O基团促进了磷灰石的沉积,进一步阻碍了腐蚀介质的侵袭。Tao等[57]采用SLM技术制备了GO/ZK30复合材料,研究了增强体GO含量对复合材料的显微组织和耐腐蚀性能的影响。结果表明,当GO含量(质量分数)从0%增加到0.9%时,MgZn2第二相颗粒分布由连续的白色网状转变为离散的点状。随着GO含量增加,析出相数量明显减少。这是因为SLM快速冷却过程中引入了大量的晶体缺陷,而GO颗粒使周围Mg基体的位错发生畸变,产生的应力场增加了Zn元素的固溶度。其中,含有0.6%GO的GO/ZK30复合材料具有最优的耐腐蚀性能,这归因于晶粒细化和析出相数量的减少。
增材制造过程中,由于镁合金粉末具有较高的化学活性,不可避免地会发生氧化。在采用激光或电子束成形时,高能量密度会破坏Mg粉表面的氧化层,从而在Mg基体中形成氧化夹杂缺陷,导致材料性能下降。为了解决上述问题,Salehi等[58]通过引入对O亲和力高于Mg的Ca,有效去除了Mg粉表面的氧化层。此外,采用BJAM技术以避免高能量密度对Mg基体产生的烧蚀,成功制备了含Ca颗粒的ZK60复合材料。结果表明,在原料Mg粉中添加纳米级Ca烧结助剂可使材料的致密化率提高25%。相比于纯Mg,含0.2%Ca的复合材料的性能显著提升,其相对密度增加约7%、拉伸强度提升约30%、抗压强度提高约15%、弹性模量提升约18%,而延伸率则增加了约185%。作为一种新兴的制造技术,增材制造技术凭借其高精度特性,可用于生产复杂结构的零部件,但其在医用镁基复合材料领域的应用研究仍处于探索阶段。
2 生物相容性增强体的选择
医用镁基复合材料中使用的增强体应具备以下特点[59]:(1) 增强体应具有生物相容性,并且最好具有促成骨性能,以便使骨折快速愈合;(2) 增强体应延缓Mg基体的降解速率以适应骨组织的愈合速率;(3) 增强体与Mg基体之间不产生剧烈的界面反应,不形成大量金属间化合物;(4) 增强体应改善Mg基体的力学性能以提供足够的力学稳定性。
目前,医用镁基复合材料中的增强体大致可分为以下几类[60]:金属氧化物,如Al2O3、ZrO2、ZnO、MgO等;磷酸钙类,如HA、β-TCP、聚磷酸钙(CPP)、氟磷灰石(FA)等;金属类,如Ti、Fe、Cu等;碳材料,如GNPs、CNTs等;以及生物活性玻璃。这些增强体通过协同强化机制,能够有效提升Mg基体的力学性能。镁基复合材料的强化机制依赖于增强体颗粒与Mg基体之间的热膨胀系数差异、弹性模量差异、纳米颗粒的沉淀强化作用,以及增强体与Mg基体间的载荷转移强化。此外,Mg基体中的细晶强化、加工硬化和固溶体强化作用也会影响复合材料的力学性能。因此,增强体的类型、尺寸、含量,以及在Mg基体中的形貌、分布和间距等参数,决定了医用镁基复合材料的力学性能和耐腐蚀性能。表1[60~72]列举了医用镁基复合材料中典型增强体类型及其对复合材料力学性能和生物学性能的影响。
表1 医用镁基复合材料中典型增强体类别及其力学性能和生物学性能[60~72]
Table 1
| Classification | Reinfor-cement | Mechanical property | Biological property | Application | Ref. |
|---|---|---|---|---|---|
| Metal oxide | Al2O3 | E = 50 GPa, σys = 176 MPa, σUCS = 486 MPa, ε = 14.0% | Improves protein adsorption, cell adhesion, and proliferation | Bone screws, bone plates, and knee prosthesis | [61] |
ZrO2 | σys = 160 MPa, σUTS = 328 MPa, ε = 11.5% | Bio-inert and nontoxic to fibroblast and blood cells, improves cell viability, and greater bone stability | Artificial knee, femoral head, and bone screw | [62] | |
| ZnO | σys = 89 MPa, σUTS = 94 MPa, ε = 1.7% | Cytocompatibility and hemocompatibility | Bone screws, bone plates | [63] | |
MgO | σys = 281 MPa, σUTS = 386 MPa, ε = 8.5% | Biodegradability, bioactivity, and good antibacterial properties | Prevent dental and orthopaedic infections | [64] | |
| Metal | Ti | σys = 134 MPa, σUCS = 418 MPa, ε = 51.0% | Biocompatibility and good antibacterial properties | Bone tissue and joint replacements | [65] |
| Fe | σUTS = 178 MPa, ε = 1.9% | Biodegradability and good antibacterial properties | Bone graft | [66] | |
Cu | σys = 237 MPa, σUTS = 286 MPa, ε = 5.4% | Good antibacterial properties | Prevent dental and orthopaedic infections | [67] | |
| Calcium | HA | σys = 245 MPa, σUCS = 388 MPa, ε = 11.0% | Nontoxic and bioactive, excellent cell proliferation and osteoblastic differentiation, and reduces the release of Mg2+ | Bone joint, bone screw, and pins | [68] |
| phosphate | |||||
| β-TCP | σUCS = 402 MPa, ε = 12.0% | Enhance bone adhesion and bone growth | Bone pin, bone screw | [69] | |
CPP | E = 48 GPa, σys = 320 MPa, σUTS = 337 MPa | Induce osteoblastic differentiation, do not cause inflammation, higher protein adsorption, and nontoxic to tissues | Bone tissue and joint replacements, dental implant | [60] | |
| FA | σys = 107 MPa, σUCS = 123 MPa, ε = 5.0% | Enhance cell viability and osteoconductivity | Bone graft | [70] | |
| Graphite | CNTs | σys = 245 MPa, σUCS = 390 MPa, ε = 15.7% | Biocompatible even in blood contact, no tissue reaction, and nontoxic to cells | Bone screw, bone plates | [71] |
| GNPs | σys = 230 MPa, σUTS = 407 MPa, ε = 13.0% | Hemocompatibility, cytocompatibility, and good antibacterial properties | Bone screw, bone plates | [72] | |
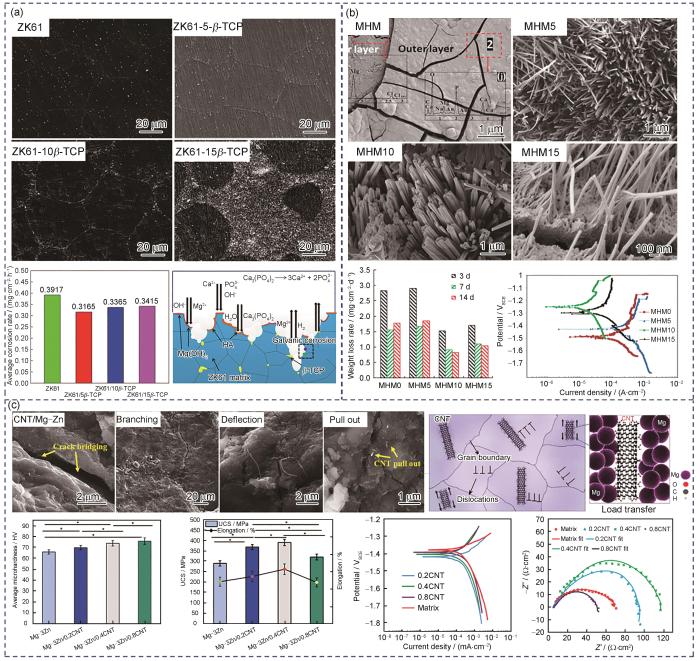
HA是磷酸钙的矿物形式,在生理环境中溶解度较低,且其化学性质和结构与天然骨相似。大量的体内/外生物学研究表明,HA具有良好的生物可降解性、生物活性和骨传导性。Del Campo等[73]通过粉末冶金法制备了HA/Mg复合材料,发现复合材料的硬度、抗压强度和Young's模量的提高主要取决于HA含量的增加,但同时HA的添加会降低复合材料的延展性。此外,与纯Mg相比,HA/Mg复合材料的降解速率减缓,耐腐蚀性能显著提高。相比于溶解度极低的HA,β-TCP具有较低的Ca / P摩尔比 = 1.5,其降解速率高于HA,因而更适用于作为生物可降解镁基复合材料的增强体。Cui等[69]通过放电等离子体烧结法制备了β-TCP/ZK61镁基复合材料,当β-TCP含量为5%时,β-TCP能够均匀分布在Mg基体晶界上,但当β-TCP含量达到15%时,则出现明显团聚现象(图5a[69])。随着β-TCP含量增加,复合材料的硬度和抗压强度提升,耐腐蚀性能也有所改善。然而,当β-TCP含量进一步增加时,β-TCP/ZK61镁基复合材料的耐腐蚀性能反而下降。原因在于,β-TCP的添加有助于在浸泡过程中形成HA和β-TCP腐蚀产物,相较于单一的Mg(OH)2层,这种腐蚀产物更具保护作用,从而提高了Mg基体的耐腐蚀性能。然而,过量的β-TCP会发生团聚,导致电偶腐蚀,从而降低耐腐蚀性能。
图5
图5
生物医用镁基复合材料的显微组织、腐蚀行为和力学性能[69,71,77]
Fig.5
Microstructure, corrosion behavior, and mechanical properties of biomedical Mg-based composites[69,71,77]
(a) SEM images, corrosion weight loss rate, and corrosion mechanism schematic of β-TCP/ZK61 composites[69]
(b) surface morphologies, corrosion weight loss rate, and electrochemical polarization curves of MgO/HA/Mg compo-sites after immersion in simulated body fluid (SBF) for 168 h[77]
(c) SEM images of crack propagation, dislocation slip schematic, mechanical properties, and electrochemical performance of CNTs/Mg-3Zn composites[71] (UCS—ultimate compressive strength; * represents P < 0.05)
相比于磷酸钙陶瓷类增强体,金属氧化物增强体具有更高的化学稳定性。其中,MgO纳米粒子具有独特的物理化学和生物学特性,包括生物可降解性、生物活性、优良抗菌性能和力学性能[74]。Tang等[75]采用热挤压法制备了MgO增强Mg-3Zn-0.2Ca镁基复合材料,用于生物医用领域。体外浸泡实验表明,添加MgO不仅能有效抑制Mg基体腐蚀产物层中裂纹的扩展,还能阻止腐蚀液体浸渗基体,显著提高腐蚀产物层的致密性。与镁合金相比,复合材料的腐蚀速率降低了30%。此外,该复合材料呈现出较低的腐蚀速率,能够为细胞黏附和分化提供安全的环境,具有更优的生物相容性。为了进一步提高HA与Mg基体的界面结合强度,Yang等[76]采用高能球磨机将MgO与HA进行混合,再使用MgO和HA的混合粉末增强WE43合金,制备了MgO/HA/WE43复合材料。TEM结果表明,HA包裹的MgO层与镁合金基体中的α-Mg晶粒形成了半共格界面,有助于形成良好的界面结合,因此阻碍了纳米HA颗粒的团聚,在Mg基体中分散均匀的HA增强体颗粒为磷灰石层的沉积提供了更多的成核位点,有利于表面钝化层的形成,从而提高了复合材料的耐腐蚀性能,自腐蚀电流密度为20.1 μA/cm2。同样地,Khalajabadi等[77]用粉末冶金法制备了纳米HA和MgO增强镁基复合材料,研究了添加MgO对HA/Mg复合材料显微组织和耐腐蚀性能的影响。结果表明,添加10%MgO后,复合材料的腐蚀速率从4.28 mm/a降低至1.06 mm/a。如图5b[77]所示,MgO/HA/Mg复合材料表面腐蚀产物主要由具有分级结构的Mg(OH)2纳米棒、HA和Ca3(PO4)2组成,且HA和Ca3(PO4)2含量随MgO含量的增加而提升。因此,添加MgO有利于改善复合材料的耐腐蚀性能。
金属类增强体因其卓越的强度、刚度以及优异的塑性变形能力,在镁基复合材料中展现出显著优势。与其他类型的增强体相比,金属类增强体通常能赋予镁基复合材料更优异的力学性能,使其在骨科承重领域的应用前景广阔。然而,由于金属类增强体(如Ti、Fe、Cu等)熔点远高于Mg,且与Mg之间固溶度极低,传统的铸造或粉末冶金方法难以成功制备出性能优良的复合材料。Meenashisundaram等[65]采用BJAM方法,首先制备出多孔Ti骨架,再将Mg熔渗入Ti骨架中,成功制备出Ti/Mg复合材料。该材料展现出与人体皮质骨相匹配的低弹性模量(约5.2 GPa)和高抗压强度(约418 MPa),并能促进成骨类SAOs-2细胞的增殖,显示出在骨科承重部位应用的潜力。Esen等[78]进一步研究了镁基复合材料的界面对降解行为的影响,采用Ti6Al4V (TC4)增强不同类型的镁合金,发现TC4增强纯Mg时,界面未析出第二相,此时由于原电池效应,Mg基体腐蚀加剧。在腐蚀过程中,产生大量H2并诱发微裂纹使基体失效。而当TC4增强AZ91镁合金时,界面生成TiAl3过渡层,有效降低了Mg基体的降解速率。尽管金属增强体能够显著改善镁基复合材料的力学性能,但由于Mg的电极电位较低,与其他金属复合后容易引发原电池效应,从而加速Mg基体的腐蚀。因此,在选择金属增强体时,需对界面电位差、增强体的种类与分布进行优化,以调控镁基复合材料的降解速率,从而满足具体应用需求。
碳材料(如CNTs和GNPs)因其高弹性模量、高力学强度,以及优异的电导率和热导率,而成为材料科学与工程领域的热点[60]。然而,碳基增强材料在生理环境中难以自然降解,需依赖人体内酶的作用加速其化学分解过程。其中,CNTs因其显著的特性,如超高强度(约150 GPa)和极高的Young's模量(约1 TPa),以及良好的生物相容性,广泛应用于医用植入材料领域[79]。Abazari等[71]采用粉末冶金技术结合挤压工艺制备了CNTs/Mg-3Zn复合材料,研究了CNTs对复合材料的抗压强度、腐蚀行为和体外生物活性的影响。结果表明,CNTs显著提升了镁合金的抗压强度,最高可达390 MPa,有望为植入后承受弯曲压力提供足够的力学支撑。如图5c[71]所示,当复合材料承受的剪应力增加时,位错能够通过切割或绕过增强体附近的局部应力集中区进行滑移,从而改善其塑性变形行为。此外,弥散分布的纳米级CNTs有助于降低Mg基体的基面织构,提高复合材料的延展性。在Mg基体中,CNTs的主要强化机制是载荷转移强化,其中最关键的因素是CNTs与基体之间强界面结合所提供的抗裂纹扩展能力。CNTs的拔出、裂纹桥接、裂纹分支和裂纹偏转是主要的增韧机制。此外,少量的CNTs (≤ 0.4%)能够改善Mg基体的耐腐蚀性能,并表现出良好的人骨肉瘤(MG-63)细胞相容性。与CNTs相比,GNPs在Mg基体中具有良好的分散性,其团聚倾向较低,进一步增强了复合材料的延展性,且GNPs具有优良的生物相容性和抗菌性能。此外,Saberi等[80]制备了具有显著抗菌性能的GNPs增强Mg-3Zn-1Ca复合材料。结果表明,GNPs/Mg-Zn-Ca复合材料能有效抑制大肠杆菌(E. coli)和金色葡萄球菌(S. aureus)的增殖。同时,低含量GNPs (≤ 1%)的复合材料对MG-63细胞表现出良好的细胞相容性,展现出GNPs增强镁基复合材料在治疗骨感染方面的潜在应用。
综上所述,镁基复合材料力学性能的强化效应主要体现在细晶强化和载荷转移强化2方面。一方面,复合材料中增强体的高强度和高硬度阻碍了Mg基体的位错运动,从而产生显著的强化作用。同时,载荷得以从基体有效转移至增强体,进一步提升了复合材料的整体强度。另一方面,增强体的引入显著细化了Mg基体的晶粒尺寸,从而提高了材料的强度。而复合材料塑性的提升主要归因于晶粒细化和增强体塑性变形的协同作用。一方面,晶粒尺寸的减小增加了单位体积内的晶粒数量,使应力分布更均匀,有效防止应力集中和随之而来的裂纹形成;另一方面,增强体通过重新分布裂纹尖端的应力,增加了阻碍裂纹扩展的驱动力。同时,增强体的引入改变了Mg基体的晶粒取向,降低了织构强度,激活了非基面滑移机制,从而显著提高了复合材料的塑性。
通常,镁合金的腐蚀行为主要由Mg与第二相之间的相互作用决定,其中Mg通常作为阳极,而第二相作为阴极。与单一镁合金不同,镁基复合材料的腐蚀过程中,Mg基体依然充当阳极,而第二相和增强体共同充当阴极。值得注意的是,增强体对镁基复合材料腐蚀行为的影响具有双重性,既可能起到积极作用,也可能带来消极影响。从积极作用来看,增强体能够显著改善镁基复合材料的耐腐蚀性能,其作用机制包括:(1) 当增强体呈网状分布时,可以充当Mg基体的物理屏障,有效提高电荷转移阻力;(2) 增强体表现为部分保护性涂层,减缓Mg基体的腐蚀速率;(3) 增强体可防止腐蚀介质中的离子渗透入Mg基体,阻碍腐蚀进程。然而,从消极影响来看,增强体在一些情况下会加剧Mg基体的腐蚀速率。例如,当增强体与Mg基体的电极电势差异过大时,会形成微电偶,导致腐蚀加速;此外,增强体与Mg基体的界面存在微裂纹或空隙时,这些缺陷为腐蚀介质的渗透提供了通道,从而诱发局部腐蚀。因此,在镁基复合材料的设计中,应综合考虑增强体的选择及其制备工艺的优化,以充分发挥其优势并尽量减少潜在的消极影响。
3 骨科临床前研究及其修复机制
3.1 骨科临床前研究
Mg是人体必需的常量元素,参与数百种生化反应,且是构成骨骼和软组织的重要成分[24]。骨科植入体不仅需要提供结构支撑还需具备促进生理修复的功能。与单一镁合金相比,镁基复合材料表现出更优异的力学性能,更符合骨植入体的需求。此外,镁基复合材料通过调控增强体来控制Mg基体的降解行为,使其与骨愈合速率相匹配,并能缓慢释放出生物活性Mg2+,模拟自然的成骨过程。Mg2+的缓慢释放可刺激外周细胞活性,调节免疫反应,增强血管内皮细胞和成骨细胞的增殖和分化,从而加速骨再生。
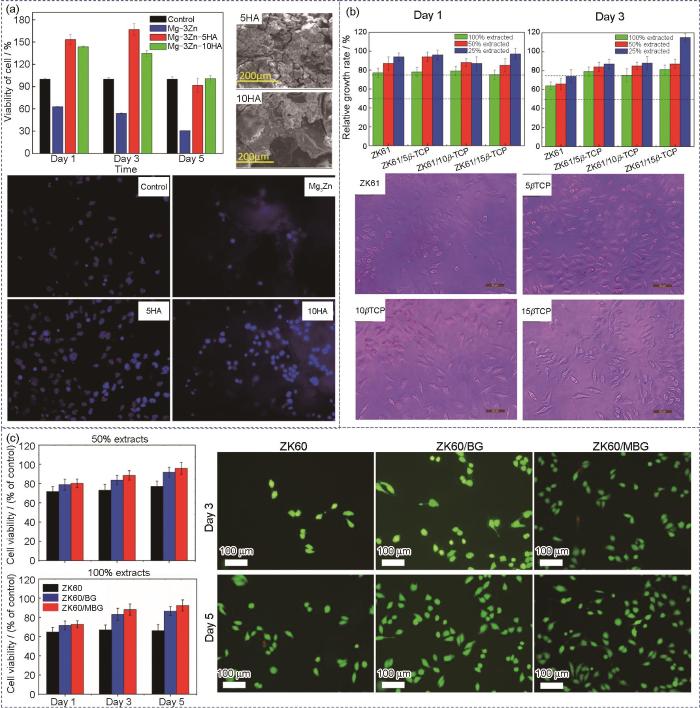
目前,如图6[69,81,82]所示,已有相关研究通过体外细胞测试评估镁基复合材料的生物相容性,发现镁基复合材料在降解过程中除了从Mg基体降解释放出Mg2+,还会从增强体中释放出特定的颗粒或阳离子,从而进一步提高其生物活性。此外,镁基复合材料还具有加速HA形成的能力,这意味着镁基复合材料具有更优异的骨整合能力。Cui等[83]通过CCK-8细胞增殖与毒性测试评估了HA/Mg复合材料的生物相容性,发现添加10%HA可以将Mg基体的腐蚀速率降低49%,该复合材料对小鼠成纤维(L-929)细胞系表现出良好的细胞相容性。Witte等[84]采用人类骨源性细胞(HBDC)、MG-63和巨噬(RAW264.7)细胞评估HA/AZ91D复合材料的生物相容性。与镁合金对照组相比,HA/AZ91D复合材料更有利于RAW264.7、MG-63和HBDC细胞的黏附和增殖。Jaiswal等[81]采用HA增强Mg-3Zn合金,长达56 d的体外浸泡测试表明,添加5%HA复合材料表面更有利于磷灰石层的沉积,Mg基体的腐蚀速率有效降低了42%。如图6a[81]所示,采用人胎儿成骨(hFOB)细胞系对HA/Mg-Zn复合材料的生物相容性进行评估,与对照组相比,添加5%和10%HA的复合材料表面的hFOB细胞活力分别增加了144%和128%,且添加5%HA的复合材料表面黏附的hFOB细胞数量最多,证明了HA增强体有利于提高复合材料的生物相容性。Cui等[69]采用四唑盐法(MTT)细胞毒性实验评估了L-929细胞在β-TCP/ZK61复合材料浸提液中的细胞活性,发现β-TCP/ZK61复合材料对L-929细胞具有良好的细胞存活率,其中具有5%β-TCP复合材料具有最好的细胞相容性(图6b[69])。Yang等[82]采用激光粉床熔融技术制备了生物活性玻璃(BG)增强ZK60镁基复合材料,通过体外模拟体液(SBF)浸泡测试评估了复合材料的降解行为,并通过体外细胞测试评估了其生物相容性。结果表明,BG增强体加速了Mg基体吸附Ca2+和HPO
图6
Fig.6
Invitro cell viability tests of Mg-based composites
(a) cell viability, SEM images, and fluorescence images of human fetal osteoblast progenitor (hFOB) cell lines cultured on HA/Mg-3Zn composites[81]
(b) cell viabilities and OM images of mouse fibroblast (L-929) cell lines cultured on β-TCP/ZK61 composites[69]
(c) cell viabilities and live/dead staining images of human osteosarcoma (MG-63) cells cultured on BG/ZK60 composites[82] (BG—bioactive glass, MBG—mesporous bioative glass)
目前,镁基复合材料在骨科领域中的应用与Mg及镁合金植入体一致,主要涉及骨组织修复和前交叉韧带重建。如图7[85~93]所示,采用动物模型评估医用镁基植入体在骨科临床应用的进展。Cheng等[85]报道高纯Mg螺钉可以提升骨形态发生蛋白-2 (BMP-2)和血管内皮生长因子(VEGF)水平,从而促进腱-骨愈合界面的愈合(图7a[85])。同时,镁基植入体降解过程中释放的Mg2+,可通过抑制基质金属蛋白酶-13 (MMP13)的表达抑制软骨吸收和肌腱移植物萎缩,有效提高愈合早期骨腱连接处的力学强度[94]。Wang等[86]研究发现,采用高纯Mg界面螺钉固定肌腱,在植入早期提高了转化生长因子β1 (TGFβ1)和血小板衍生生长因子-BB (PDGF-BB)的水平,从而改善隧道周围的新生骨形成(图7b[86])。如图7c[87]所示,Han等[87]采用高纯Mg螺钉固定股骨关节内骨折,发现与聚乳酸(PLA)螺钉相比,镁基螺钉固定的骨折区域可以形成更多的新生骨组织。Chaya等[88]采用纯Mg骨板和螺钉固定兔的尺骨骨折,由于兔子上肢不负重,观察到镁基固定系统降解后骨组织相比于对照组愈合得更好(图7d[88])。为避免内植入体早期断裂,Jähn等[89]采用镁基髓内钉(Mg-2%Ag)固定股骨干骨折,发现镁基髓内钉在降解早期表现出完整的内固定结构和良好的力学强度(图7e[89])。
图7
Fig.7
In vivo animal experiments of biomedical Mg-based composites (HP represents high-purity; black arrows represent semitendinosus, white arrow represent HP Mg-based screw; BMP-2 represents bone morphogenetic protein-2, VEGF represents vascular endothelial growth factor, GAPDH represents glyceraldehyde-3-phosphate dehydrogenase, BMSCs represent bone marrow mesenchymal stem cells)
(a) magnesium-based screw used for tendon-bone interface healing[85]
(b) Mg-based interference screw used for tendon fixation[86]
(c) Mg-based screw used for fixation of distal femur fracture[87]
(d) Mg-based screw and plate system used for ulna fracture fixation[88]
(e) Mg-based intramedullary nail used for fixation of femoral shaft fracture[89]
(f) Mg-based ring used for anterior cruciate ligament (ACL) reconstruction[90]
(g) Mg-based wire promoting meniscus regeneration[91]
(h) Mg-based suture anchor and Mg-based wire used for rotator cuff tear repair[92,93]
目前,针对镁基植入体应用于骨折固定、软骨组织修复及骨缺损再生等方向的临床前研究正日益增多。然而,镁基复合材料在这些领域中的复杂生物学机制仍需进一步深入探索与阐明。
3.2 骨修复机制
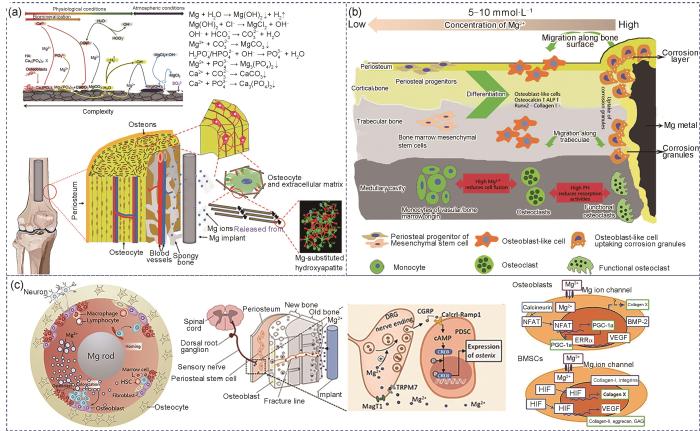
骨修复是一个涉及多种信号转导的生理过程,通常伴有神经发生和血管再生。间充质干细胞(MSCs)是重要的细胞来源,可分化为各种谱系,如成骨、软骨发生、血管生成和神经发生。如图8a[95,96]所示,镁基复合材料在生理环境下表面沉积的中间产物主要有:Mg(OH)2、MgCO3、Mg3(PO4)2、CaCO3和Ca3(PO4)2[95,96]。这些沉积产物均可以被巨噬细胞吞噬,而降解释放的Mg2+暂时储存在骨基质中,骨矿物HA是骨骼的主要成分,Mg取代植入体-新生骨界面HA中的Ca,表现出很高的生物活性以及增强的骨传导性和骨整合性[97]。随着镁基种植体的进一步降解,暂时储存在种植体周围骨基质中的Mg2+可以逐渐释放到循环系统中,而不会影响血清中Mg2+的浓度。此外,镁基植入体释放的H2可以减弱MSCs在增殖过程中的氧化应激诱导衰老过程,有助于防止骨质疏松症患者的骨质流失[98]。尽管H2能够通过周围组织快速扩散,但如果镁基植入体的降解速率过快,会导致植入体周围产生气泡,进而给患者带来健康风险[99]。因此,控制镁基复合材料的降解速率是实现镁基植入体在骨科领域广泛应用的有效策略。
图8
图8
生物医用镁基复合材料的降解行为及相关成骨机制[95,96,100~102]
Fig.8
Schematic of the degradation behavior of Mg-based composite implants under physiological conditions and the diffusion of released Mg2+[95,96] (a), reaction of Mg-based screws with surrounding bone tissue and cells after implantation[100] (ALP—alkaline phosphatase) (b), and release of Mg2+ from Mg-based intramedullary nails promoting periosteal stem cell (PSC) activation of calcitonin gene-related differentiation and enhancing hypoxia-inducible factor (HIF) expression in BMSCs[96,101,102] (c) (HSC—hematopoietic stem cell, CGRP—calcitonin gene related peptide, DRG—dorsal root ganglion, PDSC—periostem-derived stem cell, TRPM7—transient receptor potential melastatin 7, MagT1—magnesium transporter 1, cAMP—cyclic adenosine monophosphate, CREB—cAMP response element-binding protein, NFAT—nuclear factor of activated T-cells, PGC-1α—peroxisome proliferator-activated receptor gamma coactivator 1-Alpha, ERRα—estrogen-related receptor Alpha, BMP-2—bone morphogenetic protein 2, GAG—glycosaminoglycan, RANKL—receptor activator of nuclear factor-κB ligand)
骨及其周围微环境构成一个复杂的系统,由多种干细胞、祖细胞、骨细胞、成骨细胞、破骨细胞、神经纤维、内皮细胞和免疫细胞组成[96]。如图8b[100]和c[96,101~102]所示,采用体外和体内模型全面研究了镁基植入体释放的Mg2+对这些细胞的影响。参与分化的细胞类型主要取决于镁基复合材料植入的区域(干骺端或骨干)。例如,骨膜对于骨干的修复极为重要,但在干骺端区域参与较少,而MSCs对骨小梁的形成贡献更大。当采用镁基螺钉重建固定前交叉韧带时,周围的骨小梁质量得到极大改善,这很可能归因于释放的Mg2+和微碱性环境,减少了前破骨细胞的融合和破骨细胞的活性,从而抑制了破骨细胞生成(图8b[100])。如图8c[96,101,102]所示,当镁基髓内钉植入长骨(如股骨)的髓腔中时,释放的Mg2+可通过2条途径扩散:第一条是沿着Haversian管或骨折间隙,另一条是从骨髓到骨膜区域,该区域密集分布着骨膜干细胞(PDSC)和感觉神经元纤维[101]。
Yoshizawa等[102]研究了Mg2+对人骨髓间充质干细胞(hBMSCs)内信号传导机制的影响,结果表明适量Mg2+ (10 mmol/L)通过激活未分化hBMSCs中的缺氧诱导因子2α (HIF-2α)和成骨细胞中的过氧化物酶体增殖物激活受体γ辅激活因子1α (PGC-1α)实现促进COL10A1转录因子和VEGF的表达。此外,Hung等[103]发现利用10 mmol/L Mg2+培养hBMSCs时,活性β-catenin的蛋白质表达显著增加,同时免疫组织化学检测到LEF1和Dkk1 (直接受活性β-catenin控制的下游靶基因)表达增加,表明经典Wnt信号通路被激活,促进hBMSCs向成骨细胞谱系分化。而hBMSCs的分化对骨小梁的形成至关重要。值得注意的是,适量的Mg2+还能促进VEGF的表达,而VEGF在血管发育中起着核心作用,包括对骨形成至关重要的H型毛细血管。Wang等[104]制备了多孔HA/Mg复合材料应用于骨缺损填充,结果表明复合材料释放的Mg2+可通过激活Mg离子膜转运体TRPM7和MagT1诱导成骨分化,且通过激活HIF-1信号通路上调VEGF的表达,从而促进血管生成。
缺乏Mg2+会促进破骨细胞生成。Zhai等[105]发现Mg2+可抑制活化T细胞核因子(NFAT)和激活核因子(NF-κB),阻碍植入体磨损颗粒诱导的骨溶解,这表明镁基植入体具有抗破骨细胞生成的作用。骨免疫反应在骨形成和骨折愈合中尤为重要。已有研究证明,镁基植入体可诱导巨噬细胞的先天免疫反应,如图8c[96,101,102]所示。镁基植入体释放Mg2+可以促进巨噬细胞向M2型极化,从而支持成骨细胞矿化,并抑制M1型从而减轻炎症反应。这种抗炎作用可能归因于Mg2+可以提高瞬时受体电位通道M7 (TRMP7)通道的活性[106]。Liang等[107]发现Ti/Mg复合材料可以促进巨噬细胞向M2型极化,可以上调BMP-2和VEGF,并下调NF-κB信号传导。因此,镁基复合材料在骨骼生长和再生中发挥多种功能,既通过直接作用于骨骼,又通过间接作用于血管和免疫系统,为功能性骨骼再生提供了潜在的应用前景。尽管生物可降解镁基复合材料在骨科领域的相关研究逐渐增多,但目前对其如何驱动细胞级联反应并诱导相关生理功能的机制仍缺乏深入了解。因此,进一步研究并阐明其内在机制,对于优化生物可降解镁基复合材料治疗效果至关重要。
4 总结与展望
镁基材料作为极具应用前景的骨科植入器械,其制备方法、增强体类型、形貌及分布对力学性能、降解行为和生物学性能的影响至关重要。研究者已采用多种制备技术优化其力学性能,包括粉末冶金、搅拌铸造、分离熔体沉积及增材制造等。尽管这些方法已取得一定进展,但仍需进一步改进生产工艺及设备,以制备符合力学性能需求且具有高精度尺寸的骨科植入器械用镁基复合材料。目前,国内外在镁基复合材料的开发、制备和临床前研究方面取得了积极进展。大量的体外细胞实验和小型动物实验研究表明,镁基植入体对治疗骨折固定、软骨修复和骨缺损再生等骨病均具有显著疗效。通过改进制备技术、调控增强体能够进一步优化镁基复合材料的综合性能,拓宽其在复杂骨病,尤其是负重骨骼部位的应用潜力。这些研究弥补了镁基骨科植入物从基础研究到临床应用的差距,推动了其在骨科器械领域的临床应用。因此,基于国内外相关标准,对骨科植入器械用镁基复合材料的未来发展总结如下。
(1) 增强体在改善镁基复合材料力学性能和耐腐蚀性能的同时,也带来了潜在的安全隐患。未来研究应着重于增强体的降解行为及代谢机制。特别是临床前研究中,需全程追踪镁基复合材料的增强体在体内/外的降解动态。
(2) 由于生理环境复杂且多因素耦合,为准确预测镁基复合材料在复杂体内/外环境的实际降解行为,亟需建立多场耦合的体外模拟降解平台,这对全面、实时评估镁基复合材料的降解性能具有重要意义。
(3) 镁基复合材料在介导组织再生过程中涉及细胞增殖、血管再生、成骨分化、免疫反应及其与周围组织的相互作用等多种机制,但目前在该领域的研究比较有限,需进一步深入探索。
(4) 现有临床前研究多集中于非承重或低承重测试,且主要依赖于体外细胞测试和小动物模型验证。为了加快材料的临床转化,尤其在承重部位,应进行大型动物模型验证,以全面评估镁基复合材料作为骨科植入器械的应用潜力。
参考文献
Smart implants in orthopedic surgery, improving patient outcomes: A review
[J].
Titanium alloys in total joint replacement—A materials science perspective
[J].Increased use of titanium alloys as biomaterials is occurring due to their lower modulus, superior biocompatibility and enhanced corrosion resistance when compared to more conventional stainless steels and cobalt-based alloys. These attractive properties were a driving force for the early introduction of alpha (cpTi) and alpha + beta (Ti-6A1-4V) alloys as well as for the more recent development of new Ti-alloy compositions and orthopaedic metastable beta titanium alloys. The later possess enhanced biocompatibility, reduced elastic modulus, and superior strain-controlled and notch fatigue resistance. However, the poor shear strength and wear resistance of titanium alloys have nevertheless limited their biomedical use. Although the wear resistance of beta-Ti alloys has shown some improvement when compared to alpha + beta alloys, the ultimate utility of orthopaedic titanium alloys as wear components will require a more complete fundamental understanding of the wear mechanisms involved. This review examines current information on the physical and mechanical characteristics of titanium alloys used in artifical joint replacement prostheses, with a special focus on those issues associated with the long-term prosthetic requirements, e.g., fatigue and wear.
Common musculoskeletal disorders in the elderly: The star triad
[J].
A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: Current status, challenges, and future prospects
[J].
Review on titanium and titanium based alloys as biomaterials for orthopaedic applications
[J].
Research progress on the mechanical properties of the biomedical titanium alloy porous structures fabricated by 3D printing technique
[J].Porous titanium alloys have been used for biomedical implants owing to their low-modulus matching with that of human bones and interconnecting pores with suitable size, which facilitates bone in-growth and satisfies the requirement of a successful implant. Recently, additive manufacturing (3D printing) has emerged as an excellent technology for manufacturing porous implants with accurate designed pore parameters and overcoming processing difficulties caused by high melting temperatures of metals. In this paper, the microstructure and mechanical properties of porous Ti-6Al-4V, commercial pure titanium (CP-Ti), and low-modulus Ti2448 alloys produced by 3D printing, obtained mainly by the authors' group, are reviewed. For Ti-6Al-4V, its fatigue properties are affected by the type of mesh struts and post processing. The better fatigue life of CP-Ti compared to that of Ti-6Al-4V derives from its superior ductility and the strain hardening effect caused by deformation twins. The excellent fatigue life of the low-modulus Ti2448 alloy results from its superelasticity and the high toughness, which increases the crack nucleation life and fatigue crack propagation life, respectively. Future directions of corrosion-fatigue properties of materials in complex physiological environments, surface biological functionalization, and porous material of new metallic alloy systems are discussed.
3D打印医用钛合金多孔材料力学性能研究进展
[J].钛合金多孔材料具有与人体骨匹配的弹性模量,可有效解决金属植入物与人体骨弹性错配;其内部存在的大量孔隙有利于周围细胞的长入和新骨的生长,从而促进骨组织形成。近年来,增材制造(3D打印)技术被用于钛合金多孔材料制备,该方法可以精确控制孔隙参数,并且克服了因金属高熔点造成的制备困难。本文综述了作者团队在3D打印医用Ti-6Al-4V、纯Ti以及低模量钛合金多孔材料组织及力学性能的研究结果。对于Ti-6Al-4V两相合金,其疲劳性能受多孔结构设计和多种后处理的影响。纯Ti多孔材料较Ti-6Al-4V更优的疲劳寿命源于其更好的塑性和形变孪晶的应变硬化效应。低模量Ti2448合金的优异疲劳寿命则源于其超弹性提高裂纹萌生寿命,高韧性提高裂纹扩展寿命。最后展望了复杂生理环境腐蚀疲劳性能、多孔材料表面生物活化处理和新型医用金属体系多孔材料等发展方向。
Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications
[J].
A review of current challenges and prospects of magnesium and its alloy for bone implant applications
[J].Medical application materials must meet multiple requirements, and the designed implant must mimic the bone structure in shape and support the formation of bone tissue (osteogenesis). Magnesium (Mg) alloys, as a "smart" biodegradable material and as "the green engineering material in the twenty-first century", have become an outstanding bone implant material due to their natural degradability, smart biocompatibility, and desirable mechanical properties. Magnesium is recognised as the next generation of orthopaedic appliances and bioresorbable scaffolds. At the same time, improving the mechanical properties and corrosion resistance of magnesium alloys is an urgent challenge to promote the application of magnesium alloys. Nevertheless, the excessively quick deterioration rate generally results in premature mechanical integrity disintegration and local hydrogen build-up, resulting in restricted clinical bone restoration applicability. The condition of Mg bone implants is thoroughly examined in this study. The relevant approaches to boost the corrosion resistance, including purification, alloying treatment, surface coating, and Mg-based metal matrix composite, are comprehensively revealed. These characteristics are reviewed to assess the progress of contemporary Mg-based biocomposites and alloys for biomedical applications. The fabricating techniques for Mg bone implants also are thoroughly investigated. Notably, laser-based additive manufacturing fabricates customised forms and complicated porous structures based on its distinctive additive manufacturing conception. Because of its high laser energy density and strong controllability, it is capable of fast heating and cooling, allowing it to modify the microstructure and performance. This review paper aims to provide more insight on the present challenges and continued research on Mg bone implants, highlighting some of the most important characteristics, challenges, and strategies for improving Mg bone implants.© 2022. The Author(s), under exclusive licence to Islamic Azad University.
Biomedical magnesium alloys: Composition, microstructure and corrosion
[J].Magnesium alloys, with good biocompatibility and mechanical-compatibility, can be developed as next generation promising biomaterials. This paper summerizes the principle and the cutting-edge advances of alloying of magnesium alloys as degradable biomaterials. The effects of alloy elements on the material and biological properties of magnesium alloys are analyzed. The focus is laid on the influence of microstructure (grain size, secondary phase or intermetallic compound, long-period stacking ordered (LPSO) phase and quasi-crystal phase), heat treatment and surface oxide film on degradation and their critical progress on corrosion morphology and mechanism. Several outlooks on bio-magnesium alloys are proposed.
医用镁合金: 成分、组织及腐蚀
[J].镁合金具有良好的生物相容性和力学相容性,具有发展成为新一代可降解生物材料的前景。本文总结了医用镁合金合金化的原理与进展,分析了合金元素对镁合金材料学以及生物学性能的影响,重点讨论了医用镁合金显微组织(晶粒尺寸、第二相、长周期堆垛有序相(LPSO)、准晶相)、热处理和表面氧化膜对其降解行为的影响和腐蚀形态、机理方面的重要进展,并指出了医用镁合金的发展方向。
Beitrage zur technik der blutgesfass und nervennaht nebst mittheilungen die verwendung eines resorbierharen metalles in der chirurgie
[J].
Perspective: The case for an evidence-based reference interval for serum magnesium: The time has come
[J].The 2015 Dietary Guidelines Advisory Committee indicated that magnesium was a shortfall nutrient that was underconsumed relative to the Estimated Average Requirement (EAR) for many Americans. Approximately 50% of Americans consume less than the EAR for magnesium, and some age groups consume substantially less. A growing body of literature from animal, epidemiologic, and clinical studies has demonstrated a varied pathologic role for magnesium deficiency that includes electrolyte, neurologic, musculoskeletal, and inflammatory disorders; osteoporosis; hypertension; cardiovascular diseases; metabolic syndrome; and diabetes. Studies have also demonstrated that magnesium deficiency is associated with several chronic diseases and that a reduced risk of these diseases is observed with higher magnesium intake or supplementation. Subclinical magnesium deficiency can exist despite the presentation of a normal status as defined within the current serum magnesium reference interval of 0.75-0.95 mmol/L. This reference interval was derived from data from NHANES I (1974), which was based on the distribution of serum magnesium in a normal population rather than clinical outcomes. What is needed is an evidenced-based serum magnesium reference interval that reflects optimal health and the current food environment and population. We present herein data from an array of scientific studies to support the perspective that subclinical deficiencies in magnesium exist, that they contribute to several chronic diseases, and that adopting a revised serum magnesium reference interval would improve clinical care and public health.© 2016 American Society for Nutrition.
Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study
[J].Purpose: Nondegradable steel-and titanium-based implants are commonly used in orthopedic surgery. Although they provide maximal stability, they are also associated with interference on imaging modalities, may induce stress shielding, and additional explantation procedures may be necessary. Alternatively, degradable polymer implants are mechanically weaker and induce foreign body reactions. Degradable magnesium-based stents are currently being investigated in clinical trials for use in cardiovascular medicine. The magnesium alloy MgYREZr demonstrates good biocompatibility and osteoconductive properties. The aim of this prospective, randomized, clinical pilot trial was to determine if magnesium-based MgYREZr screws are equivalent to standard titanium screws for fixation during chevron osteotomy in patients with a mild hallux valgus.;Methods: Patients (n=26) were randomly assigned to undergo osteosynthesis using either titanium or degradable magnesium-based implants of the same design. The 6 month follow-up period included clinical, laboratory, and radiographic assessments.;Results: No significant differences were found in terms of the American Orthopaedic Foot and Ankle Society (AOFAS) score for hallux, visual analog scale for pain assessment, or range of motion (ROM) of the first metatarsophalangeal joint (MTPJ). No foreign body reactions, osteolysis, or systemic inflammatory reactions were detected. The groups were not significantly different in terms of radiographic or laboratory results.;Conclusion: The radiographic and clinical results of this prospective controlled study demonstrate that degradable magnesium-based screws are equivalent to titanium screws for the treatment of mild hallux valgus deformities.
Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy
[J].
Radiolucent zones of biodegradable magnesium-based screws in children and adolescents—A radiographic analysis
[J].
Fixation of unstable osteochondritis dissecans lesions and displaced osteochondral fragments using new biodegradable magnesium pins in adolescents
[J].
Magnesium research and applications: Past, present and future
[J].
Effectiveness and safety of biodegradable Mg-Nd-Zn-Zr alloy screws for the treatment of medial malleolar fractures
[J].
Structure-function integrated biodegradable Mg/polymer composites: Design, manufacturing, properties, and biomedical applications
[J].Mg is a typical biodegradable metal widely used for biomedical applications due to its considerable mechanical properties and bioactivity. Biodegradable polymers have attracted great interest owing to their favorable processability and inclusiveness. However, it is challenging for the degradation rates of Mg or polymers to precisely match tissue repair processes, and the significant changes in local pH during degradation hinder tissue repair. The concept of combining Mg with polymers is proposed to overcome the shortcomings of materials, aiming to meet repair needs from various aspects such as mechanics and biology. Therefore, it is essential to systematically understand the behavior of biodegradable Mg/polymer composite (BMPC) from the design, manufacturing, mechanical properties, degradation, and biological effects. In this review, we elaborate on the design concepts and manufacturing strategies of high-strength BMPC, the "structure-function" relationship between the microstructures and mechanical properties of composites, the variation in the degradation rate due to endogenous and exogenous factors, and the establishment of advanced degradation research platform. Additionally, the interplay among composite components during degradation and the biological function of composites under non-responsive/stimuli-responsive platforms are also discussed. Finally, we hope that this review will benefit future clinical applications of "structure-function" integrated biomaterials.© 2024 The Authors.
Biodegradable magnesium alloys promote angio‐osteogenesis to enhance bone repair
[J].
Biodegradable Mg alloys for orthopedic implants—A review
[J].The last decade has seen a significant growth in the market for alloys used for implants, especially for those intended for orthopedic implants. Research into biodegradable magnesium-based alloys has made great strides in this period, so huge progress has been made in their use in the medical industry. The important factors that led to the intensification of research in this regard, were social but also economic, wanting to improve the quality of life, by reducing the use of conventionally permanent metallic implants (stainless steel, cobalt-based alloys, and titanium alloys) which involve the second implant removal surgery and other undesirable effects (stress shielding and metal ion releases), with a negative impact on the emotional and physical condition of patients, and by significantly reducing the costs for both the patient and the health system in the field of orthopedics. This paper refers to the impact and importance of biodegradable Mg alloys, reviewing the beginning of their development, the significant characteristics that make them so desirable for such applications (orthopedic implants) but also the characteristics that must be modulated (corrosion rate and mechanical properties) to arrive at the ideal product for the targeted application. It highlights, in detail, the mechanism and aspects related to the corrosion behaviour of Mg alloys, electrochemical characterization techniques / methods, as well as strategies to improve the corrosion behaviour and mechanical properties of these types of biodegradable alloys. The means of optimization, the category and the effect of the alloying elements, the design criteria, the requirements that the implants of biodegradable alloys Mg-based must meet and the aspects related to their efficiency are also presented. Finally, the potential applications in the specialized clinics, as well as the final products currently used and made by important prestigious companies in the world are approached. (C) 2021 Chongqing University. Publishing services provided by Elsevier B.V. on behalf of KeAi Communications Co. Ltd.
Biodegradable magnesium metal matrix composites for biomedical implants: Synthesis, mechanical performance, and corrosion behavior—A review
[J].
Microstructure, mechanical, and electrochemical corrosion performance of Ti/HA (hydroxyapatite) particles reinforced Mg-3Zn squeeze casted composites
[J].
Review on manufacturability and strengthening mechanisms of particulate reinforced Mg composites
[J].
A state-of-the-art review on recent advances in the fabrication and characteristics of magnesium-based alloys in biomedical applications
[J].
Microstructure and mechanical properties of the ultra-fine grained ZK60 reinforced with low content of nano-diamond by powder metallurgy
[J].
Microstructure, mechanical property, bio-corrosion and cytotoxicity evaluations of Mg/HA composites
[J].
Graphene nanoplatelets-reinforced magnesium metal matrix nanocomposites with superior mechanical and corrosion performance for biomedical applications
[J].
Microstructures and mechanical properties of ultrafine-grained Ti/AZ31 magnesium matrix composite prepared by powder metallurgy
[J].
Simultaneous improvement of the strength and plasticity for Ti-reinforced fine-grained magnesium matrix composites prepared by powder metallurgy
[J].
Titanium particulate reinforced AZ31 magnesium matrix composites with improved ductility prepared using friction stir processing
[J].
Mechanical and corrosion behaviour of hydroxyapatite reinforced Mg-Sn alloy composite by squeeze casting for biomedical applications
[J].Magnesium alloys have gained increasing attention for biomedical applications due to their biocompatibility and the biodegradability. Hydroxyapatite (HA) is known to be a highly bioactive because of its similar chemical and crystallographic structures to bone. Therefore, HA is believed to be a potential ceramic material for the fabrication of Mg based composites, to combine the advantages of both Mg and HA. But, in general, the composites known to be more susceptible to corrosion attack than the matrix alloy. Hence, in the present work, Sn is used as an alloying element to evaluate its effect on mechanical as well as corrosion properties of Mg/HA composites. Mg with 5 wt% HA and Mg-1 wt% Sn-5 wt% HA composites were prepared separately by stir assisted squeeze casting route. The phase analysis and microstructure were characterized by X-ray diffraction (XRD) and scanning electron microscope (SEM) coupled with energy dispersive spectroscopy (EDS) respectively. Mechanical properties were evaluated by conducting the compression and micro hardness tests. Corrosion properties of as-cast composites were studied by linear polarization, Tafel and electrochemical impedance spectroscopy (EIS) techniques. The results of both XRD and SEM-EDS revealed that the main constitutional phases of as-cast Mg/HA composites were alpha-Mg and HA whereas, in Mg-Sn/HA composites, the phase Mg2Sn was observed along with fine distribution of HA particles. In both the cases, no interfacial reactions observed. The yield strength, ultimate compression strength and hardness were found to be increased with the addition of Sn in Mg/HA composites. Furthermore, the addition of Sn also played an important role in increasing the corrosion resistance of the Mg/HA composites which was attributed the refinement of grain size and the formation of Mg 2 Sn phase along the grain boundaries. Hence, it was concluded that the addition of Sn improves both mechanical and corrosion properties of Mg/HA composites. (C) 2019 Published by Elsevier B.V. on behalf of Chongqing University.
Applications of ultrasound to materials chemistry
[J].
Magnesium matrix composite reinforced by nanoparticles—A review
[J].Significant progress has been made in magnesium-based composites during recent decades, especially for the appearance of magnesium matrix composite reinforced by nanoparticles. The nanoparticles added not only exhibit a good strengthening effect, but also maintain the initial toughness of the matrix, effectively balancing the contradiction between the strength and plasticity in the traditional magnesium matrix composites. The magnesium matrix nanocomposites with excellent mechanical properties have pushed the development of magnesium matrix composites to a new stage. However, it is very difficult to disperse the nanoparticles in metal melt especially in magnesium melt which is different from other metal melts and dangerous during the cast processing. This means that the preparation of magnesium matrix nanocomposite is extremely challenging. Further, the magnesium matrix nanocomposites possess a distinctive characteristic in deformation behavior, strengthening and toughening mechanism due to their special size effect of nanoparticles. Accordingly, this review will focus on the new preparation technologies, deformation behavior, mechanical properties and strengthening and toughening mechanisms. The potential applications, development trends and future research ideas of magnesium matrix nanocomposite are also prospected. (C) 2020 Published by Elsevier B.V. on behalf of Chongqing University.
Processing, microstructure and mechanical properties of magnesium matrix nanocomposites fabricated by semisolid stirring assisted ultrasonic vibration
[J].
The microstructures and mechanical properties of low-cost Ti particles reinforced AZ81 composites
[J].
Microstructure and microhardness of SiC nanoparticles reinforced magnesium composites fabricated by ultrasonic method
[J].
Graphene nanoplatelets induced heterogeneous bimodal structural magnesium matrix composites with enhanced mechanical properties
[J].In this work, graphene nanoplatelets (GNPs) reinforced magnesium (Mg) matrix composites were synthesised using the multi-step dispersion route. Well-dispersed but inhomogeneously distributed GNPs were obtained in the matrix. Compared with the monolithic alloy, the nanocomposites exhibited dramatically enhanced Young's modulus, yield strength and ultimate tensile strength and relatively high plasticity, which mainly attributed to the significant heterogeneous laminated microstructure induced by the addition of GNPs. With increasing of the concentration of GNPs, mechanical properties of the composites were gradually improved. Especially, the strengthening efficiency of all the composites exceeded 100%, which was significantly higher than that of carbon nanotubes reinforced Mg matrix composites. The grain refinement and load transfer provided by the two-dimensional and wrinkled surface structure of GNPs were the dominated strengthening mechanisms of the composites. This investigation develops a new method for incorporating GNPs in metals for fabricating high-performance composites.
Processing, microstructure and mechanical properties of micro-SiC particles reinforced magnesium matrix composites fabricated by stir casting assisted by ultrasonic treatment processing
[J].
Processing and properties of magnesium containing a dense uniform dispersion of nanoparticles
[J].
High strength TiCp/Mg-Zn-Ca magnesium matrix nanocomposites with improved formability at low temperature
[J].
Structure-property correlation in magnesium nanocomposites synthesized by disintegrated melt deposition technique
[J].
Understanding the corrosion and bio-corrosion behaviour of magnesium composites—A critical review
[J].
Enhanced overall strength and ductility of magnesium matrix composites by low content of graphene nanoplatelets
[J].
Introducing Mg-4Zn-3Gd-1Ca/ZnO nanocomposite with compressive strengths matching/exceeding that of mild steel
[J].This work introduces Mg-4Zn-3Gd-1Ca/2ZnO (wt.%) nanocomposite fabricated using the technique of disintegrated melt deposition and extrusion. Addition of ZnO nanoparticles enhanced the compressive strengths of alloy by similar to 100 MPa. Nanocomposite samples display high strength and good ductility: 0.2% compressive yield stress of 355 MPa, ultimate compressive stress of 703 MPa, and compressive failure strain of 10.6%. The significant enhancement of compressive yield stress is mainly attributed to the grain refinement by adding nanoparticles. The strength levels exceed that of commercial magnesium alloys (i.e. WE43, WE54, ZK60, and ME21) and mild steels (i.e. S275 and S355), making Mg-4Zn-3Gd-1Ca/2ZnO a very promising material for multiple engineering and biomedical applications.
A first-time addition of selenium to a Mg-based metal matrix composite for biomedical purposes
[J].
Recent progress and perspectives in additive manufacturing of magnesium alloys
[J].
Additive manufacturing of titanium alloys for orthopedic applications: A materials science viewpoint
[J].
Additive manufacturing of ZK60 magnesium alloy by selective laser melting: Parameter optimization, microstructure and biodegradability
[J].
Processing, microstructure, and mechanical behavior of AZ31 magnesium alloy fabricated by electron beam additive manufacturing
[J].
Reactive binder jet additive manufacturing for microstructural control and dimensional stability of ceramic materials
[J].
Formability, microstructure evolution and mechanical properties of wire arc additively manufactured AZ80M magnesium alloy using gas tungsten arc welding
[J].
Research progress of additively manufactured magnesium alloys: A review
[J].Mg alloys are attractive in the fields of aerospace, automotive, and biomedical engineering, owing to the advantages of light weight, high specific strength, excellent damping property, good biocompatibility, and in vivo degradable property. However, conventional methods for manufacturing Mg alloys, such as casting and deformation processing, yield low-quality large-scale monolithic and complex structures, which hinder the applications of Mg parts. Additive manufacturing (AM) is a burgeoning alternative to manufacture monolithic parts through layer-by-layer deposition of metallic materials using 3D model data. In this paper, the latest research progress in AM of Mg alloys, which focuses on technological processes and influencing factors, macro and microstructures, mechanical properties, and corrosion properties of parts manufactured primarily by selective laser melting (SLM) and wire and arc AM (WAAM), are comprehensively reviewed. Currently, additively manufactured Mg parts with a relative density > 99% have been achievable through both SLM and WAAM after process optimization, and their mechanical properties and corrosion resistance have been comparable to those of casting and wrought parts, indicating a great potential for engineering applications. Finally, the future development trend and research direction of AM of Mg alloys are proposed from the perspectives of materials design, process improvement, and performance evaluation.
增材制造镁合金技术现状与研究进展
[J].镁合金具有轻质、比强度高、阻尼减振、生物相容性好、体内可降解等优点,在航空航天、汽车轻量化、生物医疗等领域应用潜力巨大。然而传统的镁合金铸造成形和变形加工技术在制备一体化复杂结构件上具有一定的局限性,制约了镁合金在上述领域的应用普及。增材制造是一种根据三维模型数据逐层熔化沉积的先进技术,有望成为镁合金复杂构件制备的重要技术途径。本文概述了近年来增材制造镁合金的研究进展,重点对选区激光熔化(SLM)和电弧增材制造(WAAM) 2种主要增材制造的工艺研发现状和影响因素、微观组织、力学性能及耐蚀行为进行分析与总结。研究表明,工艺优化后SLM和WAAM等技术均可获得致密度> 99%的镁合金试件,并且能够获得与传统制造镁合金相当的力学性能和耐蚀性能,增材制造镁合金表现出极大的工程应用潜力。最后,从材料优化、工艺改进及性能评价等方面对增材制造在镁合金中的未来发展趋势与研究方向进行了总结与展望。
Additive manufacturing of magnesium matrix composites: Comprehensive review of recent progress and research perspectives
[J].
Additive manufacturing of magnesium alloys by selective laser melting technology: A review
[J].Selective laser melting (SLM) additive manufacturing technology holds the broad prospect for the preparation of high-performance complex metal components owing to its high processing accuracy, short manufacturing cycle, and high material usage. Magnesium (Mg) alloys are the lightest metal structural material and provide the benefits of low density, substantial specific strength and specific stiffness, good damping and shock absorption performance, and good biodegradability. Thus, it is worthwhile to employ SLM to manufacture Mg alloys, which is predicted to widen the application scope of Mg alloys. In this study, a comprehensive review on SLM of Mg alloys focusing on the preparation of Mg alloy powders, SLM process parameters, metallurgical defects, microstructure and mechanical properties of the as-built state, post-processing, and special equipment developed for SLM of Mg alloys is given. Finally, the future development trends of the SLM of Mg alloys are explored.
镁合金选区激光熔化增材制造技术研究现状与展望
[J].选区激光熔化(SLM)增材制造技术由于其加工精度高、制造周期短、材料利用率高等优点,在制备高性能复杂金属构件方面具有广阔的应用前景。镁合金是最轻的金属结构材料,具有密度低、比强度和比刚度高、阻尼减震性能好、生物降解性良好等优点。因此,采用SLM技术制备镁合金具有重要的研究价值,有望拓宽镁合金的应用范围。本文针对镁合金SLM增材制造技术,详细介绍了镁合金粉末制备、SLM工艺参数、冶金缺陷、SLM态的显微组织和力学性能、后处理、镁合金专用SLM设备方面的研究进展,并展望了未来镁合金SLM研究的发展方向。
Selective laser melting manufactured CNTs/AZ31B composites: Heat transfer and vaporized porosity evolution
[J].
3D honeycomb nanostructure-encapsulated magnesium alloys with superior corrosion resistance and mechanical properties
[J].
Influence of graphene oxide (GO) on microstructure and biodegradation of ZK30-xGO composites prepared by selective laser melting
[J].
Enhancing densification in binder jet additive manufacturing of magnesium via nanoparticles as sintering aids
[J].
Recent developments in magnesium metal-matrix composites for biomedical applications: A review
[J].Recently, there is a growing interest in developing magnesium (Mg) based degradable biomaterial. Although corrosion is a concern for Mg, other physical properties, such as low density and Young's modulus, combined with good biocompatibility, lead to significant research and development in this area. To address the issues of corrosion and low yield strength of pure Mg, several approaches have been adopted, such as, composite preparation with suitable bioactive reinforcements, alloying, or surface modifications. This review specifically focuses on recent developments in Mg-based metal matrix composites (MMCs) for biomedical applications. Much effort has gone into finding suitable bioactive, bioresorbable reinforcements and processing techniques that can improve upon existing materials. In summary, this review provides a comprehensive overview of existing Mg-based composite preparation and their mechanical and corrosion properties and biological responses and future perspectives on the development of Mg-based composite biomaterials.
Magnesium matrix nanocomposites for orthopedic applications: A review from mechanical, corrosion, and biological perspectives
[J].Magnesium (Mg) and some of its alloys have attracted extensive interests for biomedical applications as they exhibit biodegradability and low elastic modulus that is closer to natural bones than the currently used metallic implant materials such as titanium (Ti) and its alloys, stainless steels, and cobalt-chromium (Co-Cr) alloys. However, the rapid degradation of Mg alloys and loss of their mechanical integrity before sufficient bone healing impede their clinical application. Our literature review shows that magnesium matrix nanocomposites (MMNCs) reinforced with nanoparticles possess enhanced strength, high corrosion resistance, and good biocompatibility. This article provides a detailed analysis of the effects of nanoparticle reinforcements on the mechanical properties, corrosion behavior, and biocompatibility of MMNCs as promising biodegradable implant materials. The governing equations to quantitatively predict the mechanical properties and underlying synergistic strengthening mechanisms in MMNCs are elucidated. The potential, recent advances, challenges and future research directions in relation to nanoparticles reinforced MMNCs are highlighted. STATEMENT OF SIGNIFICANCE: Critically reviewing magnesium metal matrix nanocomposites (MMNCs) for the biomedical application. Clear definitions of strengthening mechanisms using reinforcement particle in the magnesium matrix, as there were controversial in governing equations of strengthening parameters. Providing better understanding of the effect of particle size, volume fraction, interfacial bonding, and uniform dispersion of reinforcement particles on MMNCs.Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Enhancing compressive response of AZ31B magnesium alloy using alumina nanoparticulates
[J].
Mechanical, biodegradability and biocompatibility behaviour of seashell and ZrO2 particulates reinforced AZ31 Mg composites
[J].
Exploring the potential of Mg-Zn-Mn-Ca/ZnO composites as a biodegradable alternative for fracture fixation: Microstructural, mechanical, and in-vitro biocompatibility analysis
[J].
Novel microstructures inducing an excellent combination of strength and elongation in in-situ MgO/AZ31 composites
[J].
Fabrication of Ti + Mg composites by three-dimensional printing of porous Ti and subsequent pressureless infiltration of biodegradable Mg
[J].
Corrosion behavior of embedded perforated biodegradable Mg/Fe composite plate
[J].
Development of Mg/Cu nanocomposites using microwave assisted rapid sintering
[J].
Comparative property study on extruded Mg-HAP and ZM61-HAP composites
[J].
Microstructure, mechanical, corrosion properties and cytotoxicity of beta-calcium polyphosphate reinforced ZK61 magnesium alloy composite by spark plasma sintering
[J].
Novel magnesium-nanofluorapatite metal matrix nanocomposite with improved biodegradation behavior
[J].Designing and preparation of magnesium alloys with adjustable biocorrosion rates in the human body and precipitation ability of bone-like apatite layer have been of interest recently. Application of metal matrix composites (MMC) based on magnesium alloys might be an approach to this challenge. The aim of this work was fabrication and evaluation of biocorrosion and bioactivity of a novel MMC made of magnesium alloy AZ91 as matrix and fluorapatite (FA) nano particles as reinforcement. Biodegradable Magnesium-nano fluorapatite metal matrix nanocomposite (AZ91-20FA) was made via a blending-pressing-sintering method. In vitro corrosion tests were performed for evaluation of biocorrosion behavior of produced AZ91-20FA nanocomposite. The results showed that the addition of FA nano particles to magnesium alloy can reduce not only the corrosion rate in a simulated body environment but also accelerate the formation of an apatite layer.
Functionalized carbon nanotube-encapsulated magnesium-based nanocomposites with outstanding mechanical and biological properties as load-bearing bone implants
[J].
Synergetic effect of graphene nanoplatelets (GNPs) and multi-walled carbon nanotube (MW-CNTs) on mechanical properties of pure magnesium
[J].
Mechanical properties and corrosion behavior of Mg-HAP composites
[J].Mg and Mg-HAP composites containing 5, 10 and 15 wt% of hydroxyapatite have been produced following a powder metallurgy route that consists of mixing raw powders and consolidation by extrusion. The microstructure, texture, mechanical behavior and resistance to corrosion under a PBS solution have been studied. Addition of HAP increases the microhardness of the composites, however the yield strength under compression slightly decreases. Texture analyses reveal a fiber texture for pure Mg that is weakened increasing the HAP fraction. This texture promotes twinning and softening of Mg and Mg-5HAP during the initial deformation stages. Mg-10HAP and Mg-15HAP present a strain-hardening dependence showing no softening. The volume fraction of HAP particles weakens the texture and favors the activation of secondary slip systems. Corrosion experiments in PBS solution have shown that Mg-5HAP exhibits the best resistance to corrosion. Texture and porosity appear to be the main material features controlling the corrosion rates of Mg-HAP composites under the present conditions.Copyright © 2014 Elsevier Ltd. All rights reserved.
Recent advances in magnesium-magnesium oxide nanoparticle composites for biomedical applications
[J].
Effects of MgO nano particles on the mechanical properties and corrosion behavior of Mg-Zn-Ca alloy
[J].
Mechanically propelled ion exchange regulates metal/bioceramic interface characteristics to improve the corrosion resistance of Mg composite for orthopedic applications
[J].
Fabrication, bio-corrosion behavior and mechanical properties of a Mg/HA/MgO nanocomposite for biomedical applications
[J].
Corrosion behaviours of Ti6Al4V-Mg/Mg-alloy composites
[J].
Microstructure and mechanical properties of carbon nanotubes (CNTs) reinforced AZ91 matrix composite
[J].Magnesium alloys are well known for their low density, high specific strength. However, they are often limited by unsatisfactory mechanical properties. To meet the challenge of growing demand for light structural applications, metal matrix composites (MMCs) have attracted more attention. Carbon nanotubes (CNTs) have attracted much attention as the ideal reinforcements for MMCs due to their excellent mechanical strength and Young's modulus. In this work, 0.1%CNTs/AZ91 (mass fraction) magnesium matrix composites were prepared by low temperature powder metallurgy and hot extrusion. The magnesium alloy and composites were observed and analyzed by SEM, XRD and TEM. The room temperature mechanical properties of the composites were tested by Instron 5982 machine. The results showed that the CNTs distributed uniformly in the composites. The CNTs have an effect on reducing grain size, promoting precipitation of β-Mg17Al12 and weakening basal texture. The compressive strength and yield strength of the composites reached 617 and 445 MPa, which increased by 8.8% and 7.2%, respectively. The tensile strength and yield strength were 393 and 352 MPa, which 4.5% and 6.0% MPa higher than the matrix, respectively. It can be found that fine grain strengthening and load transfer play a leading role in improving the strength in the 0.1%CNTs/AZ91 magnesium matrix composites.
碳纳米管(CNTs)增强AZ91镁基复合材料组织与力学性能研究
[J].采用低温粉末冶金及热挤压工艺制备了具有超细晶组织的0.1%CNTs/AZ91 (质量分数)镁基复合材料。通过SEM、XRD、TEM对镁基复合材料的微观组织进行了表征,并对其室温力学性能进行测试。结果表明:CNTs在复合材料中分布均匀,CNTs的加入使得复合材料的晶粒尺寸从0.552 μm细化到0.346 μm,并促进了β相的析出,同时弱化了基面织构。复合材料的抗压强度和屈服强度分别达到了617和445 MPa,较基体提高了8.8%和7.2%;其抗拉强度和屈服强度分别达到了393和352 MPa,与基体相比分别提高了4.5%和6.0%。对强化机制进行分析,发现细晶强化和载荷传递是0.1%CNTs/AZ91复合材料的主要强化机制。
Magnesium-graphene nano-platelet composites: Corrosion behavior, mechanical and biological properties
[J].
Mechanical, corrosion and biocompatibility behaviour of Mg-3Zn-HA biodegradable composites for orthopaedic fixture accessories
[J].Development of biodegradable implants has grown into one of the important areas in medical science. Degradability becomes more important for orthopaedic accessories used to support fractured and damaged bones, in order to avoid second surgery for their removal after healing. Clinically available biodegradable orthopaedic materials are mainly made of polymers or ceramics. These orthopaedic accessories have an unsatisfactory mechanical strength, when used in load-bearing parts. Magnesium and its alloys can be suitable candidate for this purpose, due to their outstanding strength to weight ratio, biodegradability, non-toxicity and mechanical properties, similar to natural bone. The major drawback of magnesium is its low corrosion resistance, which also influences its mechanical and physical characteristics in service condition. An effort has been taken in this research to improve the corrosion resistance, bioactivity and mechanical strength of biodegradable magnesium alloys by synthesizing Mg-3wt% Zn matrix composite, reinforced with thermally treated hydroxyapatite(HA) [Ca(PO)(OH)], a bioactive and osteogenic ceramic. Addition of 5wt% HA is found effective in reducing the corrosion rate by 42% and improvement in the compressive yield strength of biodegradable magnesium alloy by 23%. In-vitro evaluation, up to 56 days, reveal improved resistance to degradation with HA reinforcement to Mg. Osteoblast cells show better growth and proliferation on HA reinforced surfaces of the composite. Mg-HA composite structure shows impressive potential to be used in orthopaedic fracture fixing accessories.Copyright © 2017 Elsevier Ltd. All rights reserved.
In-situ deposition of apatite layer to protect Mg-based composite fabricated via laser additive manufacturing
[J].
Effect of nano-HA content on the mechanical properties, degradation and biocompatible behavior of Mg-Zn/HA composite prepared by spark plasma sintering
[J].
Biodegradable magnesium-hydroxyapatite metal matrix composites
[J].Recent studies indicate that there is a high demand to design magnesium alloys with adjustable corrosion rates and suitable mechanical properties. An approach to this challenge might be the application of metal matrix composite (MMC) based on magnesium alloys. In this study, a MMC made of magnesium alloy AZ91D as a matrix and hydroxyapatite (HA) particles as reinforcements have been investigated in vitro for mechanical, corrosive and cytocompatible properties. The mechanical properties of the MMC-HA were adjustable by the choice of HA particle size and distribution. Corrosion tests revealed that HA particles stabilised the corrosion rate and exhibited more uniform corrosion attack in artificial sea water and cell solutions. The phase identification showed that all samples contained hcp-Mg, Mg(17)Al(12), and HA before and after immersion. After immersion in artificial sea water CaCO3 was found on MMC-HA surfaces, while no formation of CaCO3 was found after immersion in cell solutions with and without proteins. Co-cultivation of MMC-HA with human bone derived cells (HBDC), cells of an osteoblasts lineage (MG-63) and cells of a macrophage lineage (RAW264.7) revealed that RAW264.7, MG-63 and HBDC adhere, proliferate and survive on the corroding surfaces of MMC-HA. In summary, biodegradable MMC-HA are cytocompatible biomaterials with adjustable mechanical and corrosive properties.
High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF
[J].Interference screw in the fixation of autologous tendon graft to the bone tunnel is widely accepted for the reconstruction of anterior cruciate ligament (ACL), but the regeneration of fibrocartilaginous entheses could hardly be achieved with the traditional interference screw. In the present work, biodegradable high-purity magnesium (HP Mg) showed good cytocompatibility and promoted the expression of bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF), fibrocartilage markers (Aggrecan, COL2A1 and SOX-9), and glycosaminoglycan (GAG) production in vitro. The HP Mg screw was applied to fix the semitendinosus autograft to the femoral tunnel in a rabbit model of ACL reconstruction with titanium (Ti) screw as the control. The femur-tendon graft-tibia complex was retrieved at 3, 6, 9 and 12 weeks. Gross observation and range of motion (ROM) of the animal model reached normal levels at 12 weeks. No sign of host reaction was found in the X-ray scanning. The HP Mg group was comparable to the Ti group with respect to biomechanical properties of the reconstructed ACL, and the ultimate load to failure and stiffness increased 12 weeks after surgery. In the histological analysis, the HP Mg group formed distinct fibrocartilage transition zones at the tendon-bone interface 12 weeks after surgery, whereas a disorganized fibrocartilage layer was found in the Ti group. In the immunohistochemical analysis, highly positive staining of BMP-2, VEGF and the specific receptor for BMP-2 (BMPR1A) was shown at the tendon-bone interface of the HP Mg group compared with the Ti group. Furthermore, the HP Mg group had significantly higher expression of BMP-2 and VEGF than the Ti group in the early phase of tendon-bone healing, followed by enhanced expression of fibrocartilage markers and GAG production. Therefore we proposed that the stimulation of BMP-2 and VEGF by Mg ions was responsible for the fibrochondrogenesis of Mg materials. HP Mg was promising as a biodegradable interference screw with the potential to promote fibrocartilaginous entheses regeneration in ACL reconstruction. Copyright © 2015 Elsevier Ltd. All rights reserved.
Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits
[J].How to enhance tendon graft incorporation into bone tunnels for achieving satisfactory healing outcomes in patients with anterior cruciate ligament reconstruction (ACLR) is one of the most challenging clinical problems in orthopaedic sports medicine. Several studies have recently reported the beneficial effects of Mg implants in bone fracture healing, indicating the use potential of Mg devices in promoting the tendon graft osteointegration. Here, we developed an innovative Mg-based interference screws for fixation of the tendon graft in rabbits underwent ACLR and investigated the biological role of Mg-based implants in the graft healing. The titanium (Ti) interference screw was used as the control. We demonstrated that Mg interference screw significantly accelerated the incorporation of the tendon graft into bone tunnels via multiscale analytical methods including scanning electronic microscopy/energy dispersive spectrometer (SEM/EDS), micro-hardness, micro-Fourier transform infrared spectroscopy (μFTIR), and histology. Our in vivo study showed that Mg implants enhanced the recruitment of bone marrow stromal stem cells (BMSCs) towards peri-implant bone tissue, which may be ascribed to the upregulation of local TGF-β1 and PDGF-BB. Besides, the in vitro study revealed that higher Mg ions was beneficial to the improvement of capability in cell adhesion and osteogenic differentiation of BMSCs. Thus, the enhancement in cell migration, cell adhesion and osteogenic differentiation of BMSCs may contribute to an improved tendon graft osteointegration in the Mg group. Our findings in this work may further facilitate clinical applications of Mg-based interference screws for enhancing tendon graft-bone junction healing in patients indicated for ACLR.How to promote tendon-bone junction healing is one of the major challenging issues for satisfactory clinical outcomes in patients after ACL reconstruction. The improvement of bony ingrowth into the tendon graft-bone interface can enhance the tendon graft osteointegration. In this study, we applied Mg based interference screws to fix the tendon graft in rabbits and found the use of Mg screws could accelerate and significantly increase mineralized matrix formation at the tendon-bone interface in animals when compared to those with Ti screws. We elucidated the mechanism behind the favorable effects of Mg screws on the graft healing in both in vitro and in vivo studies from multiscale technologies. The optimized interface structure and function in Mg group may be ascribed to the improved cell migration capability, enhanced cell adhesion strength and promoted osteogenic differentiation ability of BMSCs under the stimuli of Mg ions degraded from implanted Mg screws. Our findings may help us broaden our thinking in the application potential of Mg interference screws in future clinical trials.Copyright © 2017 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
In vitro and in vivo studies on the degradation of high-purity Mg (99.99wt.%) screw with femoral intracondylar fractured rabbit model
[J].
In vivo study of magnesium plate and screw degradation and bone fracture healing
[J].Each year, millions of Americans suffer bone fractures, often requiring internal fixation. Current devices, like plates and screws, are made with permanent metals or resorbable polymers. Permanent metals provide strength and biocompatibility, but cause long-term complications and may require removal. Resorbable polymers reduce long-term complications, but are unsuitable for many load-bearing applications. To mitigate complications, degradable magnesium (Mg) alloys are being developed for craniofacial and orthopedic applications. Their combination of strength and degradation make them ideal for bone fixation. Previously, we conducted a pilot study comparing Mg and titanium devices with a rabbit ulna fracture model. We observed Mg device degradation, with uninhibited healing. Interestingly, we observed bone formation around degrading Mg, but not titanium, devices. These results highlighted the potential for these fixation devices. To better assess their efficacy, we conducted a more thorough study assessing 99.9% Mg devices in a similar rabbit ulna fracture model. Device degradation, fracture healing, and bone formation were evaluated using microcomputed tomography, histology and biomechanical tests. We observed device degradation throughout, and calculated a corrosion rate of 0.40±0.04mm/year after 8 weeks. In addition, we observed fracture healing by 8 weeks, and maturation after 16 weeks. In accordance with our pilot study, we observed bone formation surrounding Mg devices, with complete overgrowth by 16 weeks. Bend tests revealed no difference in flexural load of healed ulnae with Mg devices compared to intact ulnae. These data suggest that Mg devices provide stabilization to facilitate healing, while degrading and stimulating new bone formation.Copyright © 2015 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Intramedullary Mg2Ag nails augment callus formation during fracture healing in mice
[J].Intramedullary stabilization is frequently used to treat long bone fractures. Implants usually remain unless complications arise. Since implant removal can become technically very challenging with the potential to cause further tissue damage, biodegradable materials are emerging as alternative options. Magnesium (Mg)-based biodegradable implants have a controllable degradation rate and good tissue compatibility, which makes them attractive for musculoskeletal research. Here we report for the first time the implantation of intramedullary nails made of an Mg alloy containing 2% silver (Mg2Ag) into intact and fractured femora of mice. Prior in vitro analyses revealed an inhibitory effect of Mg2Ag degradation products on osteoclast differentiation and function with no impair of osteoblast function. In vivo, Mg2Ag implants degraded under non-fracture and fracture conditions within 210days and 133days, respectively. During fracture repair, osteoblast function and subsequent bone formation were enhanced, while osteoclast activity and bone resorption were decreased, leading to an augmented callus formation. We observed a widening of the femoral shaft under steady state and regenerating conditions, which was at least in part due to an uncoupled bone remodeling. However, Mg2Ag implants did not cause any systemic adverse effects. These data suggest that Mg2Ag implants might be promising for intramedullary fixation of long bone fractures, a novel concept that has to be further investigated in future studies.Biodegradable implants are promising alternatives to standard steel or titanium implants to avoid implant removal after fracture healing. We therefore developed an intramedullary nail using a novel biodegradable magnesium-silver-alloy (Mg2Ag) and investigated the in vitro and in vivo effects of the implants on bone remodeling under steady state and fracture healing conditions in mice. Our results demonstrate that intramedullary Mg2Ag nails degrade in vivo over time without causing adverse effects. Importantly, radiographs, μCT and bone histomorphometry revealed a significant increase in callus size due to an augmented bone formation rate and a reduced bone resorption in fractures supported by Mg2Ag nails, thereby improving bone healing. Thus, intramedullary Mg2Ag nails are promising biomaterials for fracture healing to circumvent implant removal.Copyright © 2016 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Magnesium ring device to restore function of a transected anterior cruciate ligament in the goat stifle joint
[J].A bioresorbable, mono-crystalline magnesium (Mg) ring device and suture implantation technique were designed to connect the ends of a transected anterior cruciate ligament (ACL) to restabilize the knee and load the ACL to prevent disuse atrophy of its insertion sites and facilitate its healing. To test its application, cadaveric goat stifle joints were evaluated using a robotic/universal force-moment sensor testing system in three states: Intact, ACL-deficient, and after Mg ring repair, at 30°, 60°, and 90° of joint flexion. Under a 67-N anterior tibial load simulating that used in clinical examinations, the corresponding anterior tibial translation (ATT) and in-situ forces in the ACL and medial meniscus for 0 and 100 N of axial compression were obtained and compared with a control group treated with suture repair. In all cases, Mg ring repair reduced the ATT by over 50% compared to the ACL-deficient joint, and in-situ forces in the ACL and medial meniscus were restored to near normal levels, showing significant improvement over suture repair. These findings suggest that Mg ring repair could successfully stabilize the joint and load the ACL immediately after surgery, laying the framework for future in vivo studies to assess its utility for ACL healing. © 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:2001-2008, 2016.© 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Local administration of magnesium promotes meniscal healing through homing of endogenous stem cells: A proof-of-concept study
[J].
Degradable magnesium alloy suture promotes fibrocartilaginous interface regeneration in a rat rotator cuff transosseous repair model
[J].
Rotator cuff repair with biodegradable high-purity magnesium suture anchor in sheep model
[J].
High-purity magnesium pin enhances bone consolidation in distraction osteogenesis via regulating Ptch protein activating Hedgehog-alternative Wnt signaling
[J].Magnesium alloys are promising biomaterials for orthopedic implants because of their degradability, osteogenic effects, and biocompatibility. Magnesium has been proven to promote distraction osteogenesis. However, its mechanism of promoting distraction osteogenesis is not thoroughly studied. In this work, a high-purity magnesium pin developed and applied in rat femur distraction osteogenesis. Mechanical test, radiological and histological analysis suggested that high-purity magnesium pin can promote distraction osteogenesis and shorten the consolidation time. Further RNA sequencing investigation found that alternative Wnt signaling was activated. In further bioinformatics analysis, it was found that the Hedgehog pathway is the upstream signaling pathway of the alternative Wnt pathway. We found that Ptch protein is a potential target of magnesium and verified by molecular dynamics that magnesium ions can bind to Ptch protein. In conclusion, HP Mg implants have the potential to enhance bone consolidation in the DO application, and this process might be via regulating Ptch protein activating Hedgehog-alternative Wnt signaling.© 2020 [The Author/The Authors].
Magnesium degradation under physiological conditions—Best practice
[J].This review focusses on the application of physiological conditions for the mechanistic understanding of magnesium degradation. Despite the undisputed relevance of simplified laboratory setups for alloy screening purposes, realistic and predictive setups are needed. Due to the complexity of these systems, the review gives an overview about technical measures, defines some caveats and can be used as a guideline for the establishment of harmonized laboratory approaches.
Biodegradable magnesium-based implants in orthopedics—A general review and perspectives
[J].
Magnesium from bioresorbable implants: Distribution and impact on the nano- and mineral structure of bone
[J].Biocompatibility is a key issue in the development of new implant materials. In this context, a novel class of biodegrading Mg implants exhibits promising properties with regard to inflammatory response and mechanical properties. The interaction between Mg degradation products and the nanoscale structure and mineralization of bone, however, is not yet sufficiently understood. Investigations by synchrotron microbeam x-ray fluorescence (μXRF), small angle x-ray scattering (μSAXS) and x-ray diffraction (μXRD) have shown the impact of degradation speed on the sites of Mg accumulation in the bone, which are around blood vessels, lacunae and the bone marrow. Only at the highest degradation rates was Mg found at the implant-bone interface. The Mg inclusion into the bone matrix appeared to be non-permanent as the Mg-level decreased after completed implant degradation. μSAXS and μXRD showed that Mg influences the hydroxyl apatite (HAP) crystallite structure, because markedly shorter and thinner HAP crystallites were found in zones of high Mg concentration. These zones also exhibited a contraction of the HAP lattice and lower crystalline order. Copyright © 2015 Elsevier Ltd. All rights reserved.
Hydrogen water consumption prevents osteopenia in ovariectomized rats
[J].
The effect of hydrogen gas evolution of magnesium implant on the postimplantation mortality of rats
[J].
High magnesium corrosion rate has an effect on osteoclast and mesenchymal stem cell role during bone remodelling
[J].The aim of this study was to gain an understanding on the collective cellular effects of magnesium (Mg) corrosion products on the behaviour of cells responsible for bone formation and remodelling. The response of mesenchymal stem cells (MSCs) and osteoclast cells to both soluble (Mg ions) and insoluble (granule) corrosion products were recapitulated in vitro by controlling the concentration of the corrosion products. Clearance of corrosion granules by MSCs was also inspected by TEM analysis at sub-cellular level. The effect of Mg corrosion products varied depending on the state of differentiation of cells, concentration and length of exposure. The presence of the corrosion products significantly altered the cells' metabolic and proliferative activities, which further affected cell fusion/differentiation. While cells tolerated higher than physiological range of Mg concentration (16 mM), concentrations below 10 mM were beneficial for cell growth. Furthermore, MSCs were shown to contribute to the clearance of intercellular corrosion granules, whilst high concentrations of corrosion products negatively impacted on osteoclast progenitor cell number and mature osteoclast cell function.
Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats
[J].Orthopedic implants containing biodegradable magnesium have been used for fracture repair with considerable efficacy; however, the underlying mechanisms by which these implants improve fracture healing remain elusive. Here we show the formation of abundant new bone at peripheral cortical sites after intramedullary implantation of a pin containing ultrapure magnesium into the intact distal femur in rats. This response was accompanied by substantial increases of neuronal calcitonin gene-related polypeptide-α (CGRP) in both the peripheral cortex of the femur and the ipsilateral dorsal root ganglia (DRG). Surgical removal of the periosteum, capsaicin denervation of sensory nerves or knockdown in vivo of the CGRP-receptor-encoding genes Calcrl or Ramp1 substantially reversed the magnesium-induced osteogenesis that we observed in this model. Overexpression of these genes, however, enhanced magnesium-induced osteogenesis. We further found that an elevation of extracellular magnesium induces magnesium transporter 1 (MAGT1)-dependent and transient receptor potential cation channel, subfamily M, member 7 (TRPM7)-dependent magnesium entry, as well as an increase in intracellular adenosine triphosphate (ATP) and the accumulation of terminal synaptic vesicles in isolated rat DRG neurons. In isolated rat periosteum-derived stem cells, CGRP induces CALCRL- and RAMP1-dependent activation of cAMP-responsive element binding protein 1 (CREB1) and SP7 (also known as osterix), and thus enhances osteogenic differentiation of these stem cells. Furthermore, we have developed an innovative, magnesium-containing intramedullary nail that facilitates femur fracture repair in rats with ovariectomy-induced osteoporosis. Taken together, these findings reveal a previously undefined role of magnesium in promoting CGRP-mediated osteogenic differentiation, which suggests the therapeutic potential of this ion in orthopedics.
Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation
[J].Magnesium alloys are being investigated for load-bearing bone fixation devices due to their initial mechanical strength, modulus similar to native bone, biocompatibility and ability to degrade in vivo. Previous studies have found Mg alloys to support bone regeneration in vivo, but the mechanisms have not been investigated in detail. In this study, we analyzed the effects of Mg(2+) stimulation on intracellular signaling mechanisms of human bone marrow stromal cells (hBMSCs). hBMSCs were cultured in medium containing 0.8, 5, 10, 20 and 100mM MgSO4, either with or without osteogenic induction factors. After 3weeks, mineralization of extracellular matrix (ECM) was analyzed by Alizarin red staining, and gene expression was analyzed by quantitative polymerase chain reaction array. Mineralization of ECM was enhanced at 5 and 10mM MgSO4, and collagen type X mRNA (COL10A1, an ECM protein deposited during bone healing) expression was increased at 10mM MgSO4 both with and without osteogenic factors. We also confirmed the increased production of collagen type X protein by Western blotting. Next, we investigated the mechanisms of intracellular signaling by analyzing the protein production of hypoxia-inducible factor (HIF)-1α and 2α (transcription factors of COL10A1), vascular endothelial growth factor (VEGF) (activated by HIF-2α) and peroxisome proliferator-activated receptor gamma coactivator (PGC)-1α (transcription coactivator of VEGF). We observed that 10mM MgSO4 stimulation enhanced COL10A1 and VEGF expression, possibly via HIF-2α in undifferentiated hBMSCs and via PGC-1α in osteogenic cells. These data suggest possible ECM proteins and transcription factors affected by Mg(2+) that are responsible for the enhanced bone regeneration observed around degradable Mg orthopedic/craniofacial devices.Copyright © 2014. Published by Elsevier Ltd.
The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway
[J].
3D printing of personalized magnesium composite bone tissue engineering scaffold for bone and angiogenesis regeneration
[J].
The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-κB and NFATc1 signaling
[J].Wear particle-induced aseptic prosthetic loosening is one of the most common reasons for total joint arthroplasty (TJA). Extensive bone destruction (osteolysis) by osteoclasts plays an important role in wear particle-induced peri-implant loosening. Thus, strategies for inhibiting osteoclast function may have therapeutic benefit for prosthetic loosening. Here, we mimicked the process of magnesium (Mg) degradation in vivo and obtained Mg leach liquor (MLL) by immersing pure Mg in culture medium. For the first time, we demonstrated that MLL suppresses osteoclast formation, polarization, and osteoclast bone resorption in vitro. An in vivo assay demonstrated that MLL attenuates wear particle-induced osteolysis. Furthermore, we found that MLL significantly inhibits nuclear factor-κB (NF-κB) activation by retarding inhibitor-κB degradation and subsequent NF-κB nuclear translocation. We also found that MLL attenuates the expression of NFATc1 at both the protein and mRNA levels. These results demonstrate that MLL has anti-osteoclast activity in vitro and prevents wear particle-induced osteolysis in vivo. Collectively, our study suggests that metallic magnesium, one of the orthopedic implants with superior properties, has significant potential for the treatment of osteolysis-related diseases caused by excessive osteoclast formation and function.Copyright © 2014 Elsevier Ltd. All rights reserved.
TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration
[J].Despite the widespread observations on the osteogenic effects of magnesium ion (Mg), the diverse roles of Mg during bone healing have not been systematically dissected. Here, we reveal a previously unknown, biphasic mode of action of Mg in bone repair. During the early inflammation phase, Mg contributes to an upregulated expression of transient receptor potential cation channel member 7 (TRPM7), and a TRPM7-dependent influx of Mg in the monocyte-macrophage lineage, resulting in the cleavage and nuclear accumulation of TRPM7-cleaved kinase fragments (M7CKs). This then triggers the phosphorylation of Histone H3 at serine 10, in a TRPM7-dependent manner at the promoters of inflammatory cytokines, leading to the formation of a pro-osteogenic immune microenvironment. In the later remodeling phase, however, the continued exposure of Mg not only lead to the over-activation of NF-κB signaling in macrophages and increased number of osteoclastic-like cells but also decelerates bone maturation through the suppression of hydroxyapatite precipitation. Thus, the negative effects of Mg on osteogenesis can override the initial pro-osteogenic benefits of Mg. Taken together, this study establishes a paradigm shift in the understanding of the diverse and multifaceted roles of Mg in bone healing.
Enhancing the immunomodulatory osteogenic properties of Ti-Mg alloy by Mg2+-containing nanostructures
[J].