碳钢在大气环境中发生腐蚀时,其表面通常伴随着锈层的形成过程,锈层及其成分和结构的演变是构成碳钢腐蚀行为不可或缺的要素[1~3]。碳钢的锈层通常由Fe3O4、α-FeOOH、γ-FeOOH、γ-Fe2O3等物相组成[4]。γ-FeOOH结构疏松多孔,致密性较差,可能会对锈层的保护性产生不利的影响[5];α-FeOOH结构致密,是锈层中最稳定的物相,是保护性锈层的主要构成相[6];Fe3O4和γ-Fe2O3都是铁磁性物质,具有较好的致密性和稳定性,通常也被认为具有保护性[7]。上述各物相在锈层中的占比和分布直接决定了整个锈层的结构特性,同时锈层的物相组成与锈层结构互相影响,并最终影响锈层的保护性。
碳钢表面锈层的成分和结构主要受环境因素和材料本体的影响。例如,碳钢服役环境条件不同,其表面的成锈行为存在显著差异。汪川等[8]发现,在工业大气中,SO2会加速α-FeOOH的生成,导致低合金钢的腐蚀速率快速下降,而在海洋大气中,高浓度Cl-导致基体-锈层界面之间的Fe3O4呈强磁性,无法形成致密稳定的锈层。Pan等[9]研究了碳钢在工业环境下锈层演化的情况,发现锈层主要由Fe3O4、α-FeOOH、γ-FeOOH等物质构成,呈现双层结构,外层松散,内层致密,外层结构在12个月后消失。此外,合金元素也会对锈层造成显著的影响,耐候钢的锈层比普碳钢的锈层具有保护作用的根源在于耐候钢中含有 Cu、Cr、Ni、稀土等合金元素,这些合金元素可以使耐候钢表面生成致密的保护性锈层。除了受到上述因素的影响,力学因素对碳钢锈层的影响也不容忽视。在实际服役过程中,碳钢普遍承受一定应力的作用。在应力和腐蚀介质的协同作用下,腐蚀损耗的能量由碳钢应变时释放的应变能和电化学过程释放的能量共同提供,从而加速碳钢的腐蚀速率,促进锈层的生长[10~12]。根据Evans[13]的理论,由于机械变形而导致的原子排列混乱可以促进原子从金属中分离。Kim等[14]认为,变形可以在钢上诱导局部阳极溶解,导致腐蚀电位降低和电流密度增加。另一方面,在钢结构的实际使用条件下,弹性范围内的拉应力对腐蚀和开裂失效的影响也可能是一个重要问题[15]。此外,应力可能会对锈层的结构和成分产生影响。Gao等[16]研究了低碳贝氏体耐候钢在含Cl-环境中的腐蚀行为,当施加的载荷增加时,锈层中出现裂纹,锈层呈现多孔状,这意味着施加弹性拉应力降低了锈层对Cl-扩散的阻力,锈层中阴离子的选择性增强。Zhao等[17]研究发现,基体弹性拉应力对S450EW耐候钢在盐雾中形成锈层的成分和结构有显著影响:弹性拉应力通过磁弹效应削弱锈层中磁性成分与基体的磁力作用,从而削弱锈层的成分偏聚和结构分层。然而,该研究忽略了弹性拉应力通过电化学过程作用于耐候钢锈层的可能性,而基体-锈层界面的电化学过程对锈层成分和结构的演变有十分重要的影响。
本工作从电化学过程的角度着手,通过中性盐雾实验结合四点弯曲法进行弹性拉应力下的预腐蚀成锈,并结合2种应力加载方式(试样在中性盐雾实验和电化学测试时一直加载应力;试样只在电化学测试加载应力)的电化学测试结果,研究了基体弹性拉应力对碳钢锈层的成分、结构和电化学特性的影响及其电化学机制。
1 实验方法
1.1 实验材料和应力加载
为了排除合金元素对锈层的影响,选用材料为Q235碳钢,主要化学成分(质量分数,%)为:C 0.14,Mn 0.55,Si 0.19,S 0.002,P 0.026,Fe余量。Q235碳钢的屈服强度(σs)为273 MPa,抗拉强度(σb)为413 MPa,延伸率(δ)为33.5%。
所有试样尺寸均为110 mm × 15 mm × 2 mm,采用系列SiC砂纸将试样表面逐级打磨至2000号,然后用蒸馏水和乙醇清洗,并干燥备用。为了凸显有无应力作用下成锈后基体电化学行为的不同,进而反推应力对锈层电化学特性的影响规律和机制,采用A、B 2种应力加载方式:A是指在预腐蚀成锈过程中(15 d中性盐雾实验)和电化学测试过程中,试样一直处于应力加载状态;B是指在预腐蚀成锈过程中(15 d中性盐雾实验),试样不加载应力,而在电化学测试前,对试样进行应力加载。相应地,将试样分为A、B 2组:A组试样按用途分为失重分析和锈层物相成分分析试样、电化学测试试样以及锈层截面形貌观察试样;B组试样只用于电化学测试。
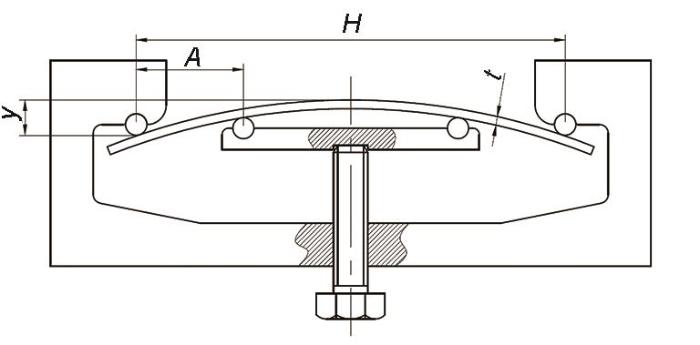
A、B 2组试样的应力加载水平包括0σs、0.5σs、0.8σs和0.95σs,分别对应于0、137、218和260 MPa。采用如图1所示的四点弯曲装置加载应力,四点弯曲夹具的设计参考GB/T 15970.2—2000《金属和合金的腐蚀-应力腐蚀试验-第2部分:弯梁试样的制备和应用》。夹具外支点间的距离(H)为100 mm,相邻内外支点间的距离(A)为25 mm,通过旋转螺杆调节两内支点的高度,从而可实现对试样挠度(y)的调节。
图1
图1
四点弯曲加载装置示意图
Fig.1
Schematic of four-point bending device (H—distance between the outer fulcrums of the fixture, A—distance between adjacent inner and outer fulcrums, y—sample deflection, t—sample thickness)
在弹性形变范围内,两内支点间的凸面承受均匀一致的弹性拉应力,该弹性拉应力(σ)由下式计算:
式中,E为材料的弹性模量,对Q235碳钢而言,其值为201 GPa;t为试样厚度,本研究中为2 mm。根据目标应力,可通过
1.2 中性盐雾实验和失重分析
盐雾实验在可控制温度和喷雾时间的CH150盐雾箱内进行,参照国标GB/T10125—2012中关于中性盐雾实验法的相关规定进行实验。实验温度35℃,盐雾所用溶液为5%NaCl (质量分数),盐雾平均沉降率为(187.5 ± 62.5) mL/(m2·h)。每组设置3个平行试样,实验前,将处理好的试样称重,记录重量m0。针对A组中的失重分析试样,除周期15 d外,还设置周期为1、3、6和9 d的盐雾实验以记录失重和锈层表面形貌随时间的变化规律。每个周期结束后,采用去离子水冲洗掉试样表面的浮锈,然后进行表面宏观形貌观察。然后,置于鼓风干燥箱内干燥24 h,干燥温度为35℃。干燥后,用刀片小心剥离锈层(避免伤及基体),保存剥离下来的锈层用于物相成分分析。最后,利用除锈液和超声波清洗除去试样表面的剩余锈层。除锈液根据国标GB/T 16545—2015推荐,由500 mL HCl + 500 mL H2O + 3.5 g C6H12N4组成。除锈后,将试样清洗、吹干,然后称重,记录重量m,试样经中性盐雾实验后的质量损失即为m0 - m,用msteel表示。
1.3 电化学测试
A和B组中的电化学测试试样经盐雾实验15 d后,使用V3电化学工作站对其进行电化学测试,包括开路电位(OCP)测试和电化学阻抗谱(EIS)测试。采用三电极体系:待测试的试样为工作电极,饱和甘汞电极为参比电极,Pt片为辅助电极,所用介质为5%NaCl溶液。OCP测试时间为3600 s,每秒采点1次,取最后10 min的数据计算OCP的平均值和方差。EIS测试频率范围为100 kHz~10 mHz,交流激励信号幅值为±10 mV,扫描步长为12个点每10倍频。
1.4 锈层的截面形貌观察
A组中锈层截面形貌观察试样经盐雾实验15 d后,将其从四点弯曲夹具中取出,清除掉试样表面的硅橡胶,置于35℃的鼓风干燥箱内干燥24 h,然后利用环氧树脂和PVC管对试样进行密封,以保护锈层在后续的磨抛中不被破坏。密封完成后将试样沿观察面截断,用砂纸逐级将截面打磨至2000号,然后用2.5 μm的SiC抛光膏抛光。抛光后,洗净吹干,以备观察。采用Flex型扫描电子显微镜(SEM)观察锈层的截面形貌,结合能谱仪(EDS)对锈层截面上的Fe、O、Cl等元素的分布进行分析。
1.5 锈层成分分析
将剥离的锈层研磨,并过筛成粒度小于44 μm的粉末。采用D8 Advance X射线衍射仪(XRD)分析锈层物相,测试时使用Cu靶Kα 射线,扫描角度为10°~70°,扫描速率为2°/min。
2 实验结果
2.1 腐蚀失重
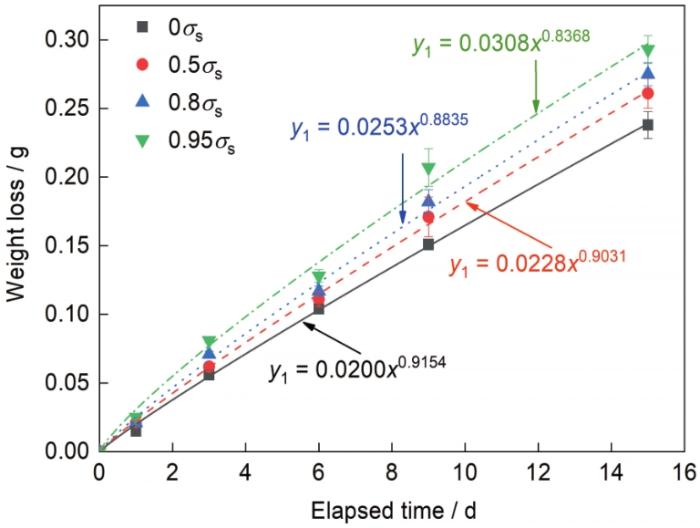
图2是不同弹性拉应力水平下Q235碳钢的质量损失随腐蚀时间的变化曲线,利用经验公式y1 = axb [3]对腐蚀失重数据进行拟合,其中y1是试样在单位面积内的质量损失,x为腐蚀时间,a和b为具有腐蚀动力学意义的参数。a代表初始的腐蚀速率,a越大表示初期腐蚀越快;b代表腐蚀速率发展的趋势,b大于1意味着锈层对基体没有保护作用,b小于1表示锈层对基体有保护作用,且b越小说明锈层的保护作用越大[9,18]。拟合得到的结果如表1所示。可知,a随应力水平的提高而增大,表明应力水平越高试样初期的腐蚀速率越快;所有应力水平下的b均小于1,并且随着应力水平的提高呈减小趋势,说明锈层对基体产生了一定的保护作用,并且这种保护性随着应力水平的提高而增强。
图2
图2
不同弹性拉应力水平下Q235碳钢在5%NaCl盐雾中的质量损失随时间的变化
Fig.2
Time dependences of the weight loss of Q235 carbon steel under various elastic tensile stress levels in 5%NaCl salt spray (σs—yield strength)
表1 不同弹性拉应力水平下Q235碳钢在5%NaCl盐雾中的腐蚀动力学拟合结果
Table 1
| Stress level | a | b | R2 |
|---|---|---|---|
| 0σs | 0.0200 | 0.9154 | 0.9987 |
| 0.5σs | 0.0228 | 0.9031 | 0.9981 |
| 0.8σs | 0.0253 | 0.8835 | 0.9962 |
| 0.95σs | 0.0308 | 0.8368 | 0.9898 |
2.2 锈层形貌
在中性盐雾实验1、3、6、9和15 d时,A组失重分析试样表面的宏观形貌如图3所示。可以看出,在1~3 d时,锈层未完全覆盖试样表面,不同弹性拉应力水平下锈层表面均呈橘红色,锈层边缘处呈现黑褐色;6~15 d时,随着应力水平的提高和腐蚀时间的延长,锈层表面红色物质逐渐减少,黑褐色物质逐渐增多,尤其是黄色虚线区域内,锈层表面大部分为黑色物质。这种颜色变化与不同腐蚀阶段锈层的成分演变有关,其中黑色物质被认为是磁铁矿(Fe3O4),橘红色物质被认为是纤铁矿(γ-FeOOH)或者针铁矿(α-FeOOH)[19]。锈层表面颜色的变化说明随着腐蚀时间的延长和弹性拉应力水平的提高,锈层中Fe3O4的含量可能在不断增加,γ-FeOOH或α-FeOOH的含量不断减少,各物相含量的具体变化情况在下文进一步分析。
图3
图3
不同弹性拉应力水平下Q235碳钢在5%NaCl盐雾中暴露不同时间后的表面宏观形貌
Fig.3
Surface morphologies of Q235 carbon steel under various elastic tensile stress levels after exposed in 5%NaCl salt spray for 1 d (a1-a4), 3 d (b1-b4), 6 d (c1-c4), 9 d (d1-d4), and 15 d (e1-e4) (a1-e1) 0σs (a2-e2) 0.5σs (a3-e3) 0.8σs (a4-e4) 0.95σs
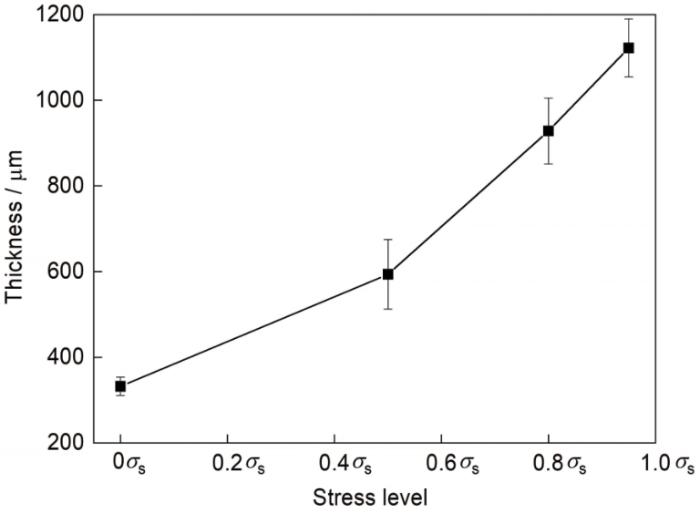
图4所示为不同弹性拉应力水平下Q235碳钢经中性盐雾实验15 d后的锈层截面形貌及元素分布。可见,随应力水平的提高,锈层厚度显著增加。对不同应力水平下的锈层厚度进行测量,测量结果示于图5中。从图5可知,0.95σs应力水平下锈层厚度约为无应力下的3倍。从图4还可见,锈层内部的孔隙尺寸和数量随应力水平提高而增加。一方面,锈层厚度的增加有利于提高锈层对基体的隔离性能;但另一方面,锈层中孔隙为O和离子的扩散提供了通道,使它们容易穿透锈层[20]。这2方面的因素会对锈层下钢基体的腐蚀行为产生复杂影响:前者可能通过隔离作用减缓腐蚀,但也可能增强锈层下环境的闭塞性,造成离子浓度升高和环境酸化;后者则有利于减弱锈层下环境的闭塞性,但同时会促进O的扩散,促进阴极吸氧反应。从Cl元素的分布上看,随着应力水平的提高,Cl等元素在锈层中的分布无明显规律,Cl并未在锈层内侧发生偏聚。这说明尽管随着应力水平提高,锈层厚度增加,但并未造成锈层下环境闭塞性的增强。因此,弹性拉应力对锈层保护性的影响并非与锈层厚度和致密性呈单调相关,还需结合电化学测试结果进行具体分析。
图4
图4
不同弹性拉应力水平下Q235碳钢在5%NaCl盐雾中暴露15 d后的锈层截面形貌和元素分布
(a) 0σs (b) 0.5σs (c) 0.8σs (d) 0.95σs
Fig.4
Section morphologies and corresponding element distributions of the rust layers of Q235 carbon steel under various elastic tensile stress levels after exposed in 5%NaCl salt spray for 15 d
图5
图5
Q235碳钢在5%NaCl盐雾中暴露15 d后锈层的厚度随钢基体所受弹性拉应力水平的变化
Fig.5
Variations of the rust thickness with the elastic tensile stress level for Q235 carbon steel after exposed in 5%NaCl salt spray for 15 d
2.3 锈层成分
不同弹性拉应力作用下Q235碳钢形成锈层的XRD谱如图6a所示。使用Jade软件标定后可知,各应力水平下锈层中主要包含针铁矿(α-FeOOH)、纤铁矿(γ-FeOOH)、磁铁矿或磁赤铁矿(Fe3O4/γ-Fe2O3)等物相。其中,γ-FeOOH和Fe3O4/γ-Fe2O3的峰形较尖锐,而α-FeOOH的峰形相对平缓,这表明前2者的结晶完成度较后者高。不同弹性拉应力水平下,锈层的物相组成无显著区别,但是各物相的相对含量有显著不同。需要注意的是,由于XRD无法区分磁铁矿和磁赤铁矿,其在图谱中被标记为同一相,以M(Fe3O4/γ-Fe2O3)表示。采用参考强度比(RIR)半定量分析法[21]对各相含量进行了分析,分析结果如图6b所示。可见,在盐雾实验15 d后,各应力水平下形成的锈层中Fe3O4/γ-Fe2O3占比最多,超过50% (质量分数)。随着应力由0σs增大至0.95σs,Fe3O4/γ-Fe2O3的质量分数由53%减小至约46%,α-FeOOH的质量分数由约30%减小至约23%,γ-FeOOH的质量分数由不到17%增大至约31%。在锈层的物相中,α-FeOOH有利于提高锈层的致密性,而γ-FeOOH则相反[22]。因此,由2种物相的质量分数随应力水平的变化趋势可知,弹性拉应力可能减弱了γ-FeOOH向α-FeOOH的转化,从而导致锈层的致密性下降。
图6
图6
不同弹性拉应力水平下Q235碳钢在5%NaCl盐雾中暴露15 d后锈层的XRD谱和物相组成
Fig.6
XRD spectra (a) and phase compositions (b) in the rust layer of Q235 carbon steel under various elastic tensile stress levels after exposed in 5%NaCl salt spray for 15 d
2.4 锈层的质量和密度
一般而言,锈层的致密性与其密度直接相关,且呈正相关。通过计算不同弹性拉应力水平下锈层的密度,可探究弹性拉应力对锈层致密度和结构的影响。基于上述腐蚀失重和物相成分结果,对锈层的质量进行计算。
首先,锈层中各物相的质量可由锈层的总质量(mrust)与该物相的质量分数(r)相乘获得,即:
式中,m1、m2和m3分别代表γ-FeOOH、α-FeOOH和Fe3O4 3种物相的质量;r1、r2和r3分别为γ-FeOOH、α-FeOOH和Fe3O4/γ-Fe2O3 3种物相的质量分数。
根据Fe的相对原子质量与各物相的相对分子质量之比,可求得各物相中Fe的质量。γ-FeOOH、α-FeOOH和Fe3O4/γ-Fe2O3 3种物相中Fe的质量之和约等于Q235碳钢的质量损失(msteel):
式中,Ar是Fe的相对原子质量,其值为56;M1、M2和M3分别为γ-FeOOH、α-FeOOH和Fe3O4/γ-Fe2O3物相的相对分子质量,其值分别为89、89和232。将式(
结合锈层的截面厚度和试样暴露表面积,由下式估算锈层的密度:
图7
图7
Q235碳钢在5%NaCl盐雾中暴露15 d后锈层的单位面积质量和密度随钢基体所受弹性拉应力水平的变化
Fig.7
Variations of mass per unit area and density of the rust layer with the elastic tensile stress level for Q235 carbon steel after exposed in 5%NaCl salt spray for 15 d
2.5 电化学结果
图8为加载方式A和B下Q235碳钢在不同水平弹性拉应力作用下经中性盐雾实验15 d后的OCP。在加载方式A和B下,OCP随应力水平提高分别呈升高和降低趋势,这说明基体弹性拉应力对锈层的电化学特性存在显著影响。在加载方式B下,由于弹性拉应力对试样的作用只发生在电化学测试期间,因而加载方式B下OCP的变化主要反映了弹性拉应力对电极表面电化学反应的作用,即弹性拉应力提高了钢基体的电化学活性,促进了阳极溶解。在加载方式A下,弹性拉应力对试样的作用存在于锈层形成期间和电化学测试期间,因此A加载方式下OCP的变化反映了弹性拉应力对锈层和电极表面电化学反应2方面的综合影响。对比A和B加载方式下OCP的变化趋势可知,弹性拉应力通过影响锈层而提高OCP的趋势覆盖了通过提高钢基体电化学活性降低OCP的趋势。结合上述关于锈层截面形貌和锈层密度的结果(图4和7),可知弹性拉应力对OCP的提高可来自2方面:一方面,弹性拉应力促进锈层质量增加,导致锈层界面的电压差增加,从而提高OCP;另一方面,弹性拉应力使锈层疏松度增加,促进锈层内外物质扩散,从而减轻锈层内侧环境的酸化和离子浓度升高。
图8
图8
加载方式A和B下Q235碳钢在5%NaCl盐雾中暴露15 d后测得的开路电位随钢基体所受弹性拉应力水平的变化
Fig.8
Variations of the open circuit potential measured under the loading methods A and B with elastic tensile stress level for Q235 carbon steel in 5%NaCl salt spray for 15 d (Method A—during the salt spray test period of 15 d and the electrochemical test process, the sample kept the stress loading state; Method B—no stress was loaded on the sample within 15 d of the salt spray test period, and the stress was loaded on the sample during electrochemical test after the salt spray test)
图9展示了A和B 2种加载方式的不同应力水平下Q235碳钢盐雾腐蚀15 d时测得的EIS曲线。从图9a和d可见,A和B加载方式的不同应力水平下Nyquist曲线在低频区域均呈现不同倾斜程度的扩散尾,这表明不同应力水平下Q235碳钢形成的锈层对电化学过程均起到不同程度的扩散控制作用[15]。而在中高频区域,A和B加载方式的不同应力水平下Nyquist曲线均表现出重叠的压缩半圆容抗弧特征,这种特征是双电层电容和表面腐蚀产物膜电容叠加作用的结果[23],如图9b和e所示。从相位角图(图9c和f)也可见,各条件下的EIS曲线均存在至少2个时间常数。此外,各曲线相位角的最大值均远低于90,这是由于电极的不均匀性、孔隙率、质量传输和弛豫效应而导致的对理想电容器特性的偏离[24,25]。
图9
图9
不同弹性拉应力水平的加载方式A和B下Q235碳钢在5%NaCl盐雾中暴露15 d后的EIS
Fig.9
Nyquist (a, d), Bode-impedance modulus (b, e), and Bode-phase angle (c, f) plots of Q235 carbon steel with loading modes A (a-c) and B (d-f) under various elastic tensile stress levels after exposed in 5%NaCl salt spray for 15 d (Zim—imaginary part of impedance, Zre—real component, |Z|—impedance modulus)
表2展示了图9中各EIS曲线的拟合结果。从表2可见,各条件下拟合的卡方值(χ2)均在10-4数量级,结合图9所展示拟合曲线与实测曲线的吻合程度,说明拟合结果准确可信。同时,各元件的拟合值与文献[27,28]报道的类似材料和体系的拟合值在数量级上接近,这进一步说明了拟合结果的准确性。基于上述分析和锈层截面形貌观察,采用如图10所示的等效电路图对上述EIS曲线进行了拟合。在图10中,Qrust是锈层的常相位角元件,代表整个锈层的电容特性;Rs是辅助电极与锈层之间的溶液电阻;Rrust是锈层电阻,代表离子在锈层中电迁移的阻力,与锈层的厚度和致密度相关;Qct是双电层恒相元件;Rct是电荷转移电阻,代表双电层界面电荷传递的快慢,在本工作中反映了锈层下钢基体的电化学反应活性[26];W为半无限扩散阻抗,代表反应物或生成物扩散至或离开双电层界面的阻力。
表2 不同弹性拉应力水平的加载方式A和B下Q235碳钢在5%NaCl盐雾中暴露15 d后EIS的拟合结果
Table 2
| Mode | Stress | Rs | Qrust (Y0) | nrust | Rrust | Qct (Y0) | nct | Rct | W | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| level | Ω·cm2 | 10-2 Ω-1·cm-2·s n | Ω·cm2 | 10-2 Ω-1·cm-2·s n | Ω·cm2 | 10-2 Ω-1·cm-2·s0.5 | 10-4 | |||
| A | 0σs | 11.60 | 1.05 | 0.52 | 10.51 | 0.17 | 0.68 | 53.21 | 6.88 | 7.21 |
| 0.5σs | 9.59 | 0.52 | 0.49 | 11.25 | 0.62 | 0.60 | 46.28 | 6.52 | 1.91 | |
| 0.8σs | 10.83 | 0.24 | 0.43 | 12.59 | 1.38 | 0.62 | 32.98 | 5.98 | 4.61 | |
| 0.95σs | 9.94 | 0.14 | 0.37 | 14.46 | 2.36 | 0.57 | 29.04 | 5.46 | 2.01 | |
| B | 0σs | 11.60 | 1.05 | 0.52 | 10.51 | 0.17 | 0.68 | 53.21 | 6.88 | 7.21 |
| 0.5σs | 8.76 | 0.36 | 0.51 | 9.94 | 1.46 | 0.54 | 35.43 | 6.64 | 1.78 | |
| 0.8σs | 10.19 | 0.32 | 0.48 | 9.07 | 2.68 | 0.48 | 21.62 | 6.24 | 3.16 | |
| 0.95σs | 12.16 | 0.51 | 0.47 | 8.83 | 2.78 | 0.44 | 16.48 | 6.13 | 2.08 |
图10
图10
Q235碳钢在5%NaCl盐雾中暴露15 d后锈层界面的等效电路图
Fig.10
Equivalent electric circuit of the rust layer interface on Q235 carbon steel after exposed in 5%NaCl salt spray for 15 d
图11
图11
加载方式A和B下等效电路中电荷转移电阻(Rct)、锈层电阻(Rrust)与电容弥散系数(nrust)、扩散阻抗(W)随弹性拉应力水平的变化趋势
Fig.11
Variations of Rct, Rrust (a) and nrust, W (b) in the equivalent electric circuit with elastic tensile stress levels under loading modes A and B
由图11a还可以看出,随钢基体所受应力水平的提高,加载方式A下Rrust逐渐增大,而加载方式B下Rrust略微减小。由于Rrust主要与锈层厚度和致密度相关,结合锈层的截面形貌和密度结果可知,尽管锈层的致密性随应力水平提高而降低,但同时锈层厚度也随之增加,后者对Rrust的增加效果超过了前者对Rrust的减小效果,因而基体受弹性拉应力作用下形成锈层的Rrust从整体上表现出随应力水平增加而增大的趋势。由于加载方式B下的锈层实际为试样在无应力下形成的,因而加载方式B下Rrust随应力水平提高而减小这一趋势,实际是由电化学测试前弹性拉应力的加载对锈层的破坏所致,但由Rrust减小的幅度可知弹性拉应力对锈层的破坏作用较小。
由图11b可知,A、B 2种加载方式下nrust均远小于1,且随钢基体所受应力水平增加而减小。nrust的大小反映的是锈层偏离理想电容的弥散特性:其值越接近1,锈层的电学特性越接近理想电容;其值越接近0,锈层的电学特性越接近电阻。同时,nrust的大小也一定程度上反映了锈层作为电容介电质的均一性,这体现在2个方面:一方面,锈层的主要成分Fe3O4/γ-Fe2O3的质量分数随应力水平增加而减小(图6b),锈层的成分均一性下降;另一方面,锈层的致密度随应力水平增加而降低(图4和7),锈层的结构均一性下降。因此,加载方式A下nrust随应力水平的变化趋势与锈层的成分和致密度随应力水平的变化趋势体现了一致性。对于加载方式B下的锈层,由于弹性拉应力未参与锈层的形成过程,因而加载方式B下nrust随应力水平增加而减小的趋势显著弱于加载方式A。
由图11b还可知,A、B 2种加载方式下W均随钢基体所受应力水平增加而减小。这说明反应物或生成物穿过锈层的扩散阻力随应力水平增大而减小。显然,这是由于锈层的致密度随应力水平增加而降低所致。同样地,由于弹性拉应力未参与加载方式B下试样锈层的形成过程,因而加载方式B下W随应力水平增加而减小的趋势显著弱于加载方式A。
3 分析讨论
3.1 弹性拉应力对锈层成分的影响
弹性拉应力对Q235碳钢锈层成分的影响与锈层形成的电化学过程紧密相关。碳钢置于5%NaCl盐雾中后,电化学反应迅速发生,Fe被氧化为Fe2+ [29]:
随后FeOH+与
在锈层的稳定化过程中,部分γ-FeOOH溶解成无定形的FeO x (OH)3 - 2x,通过固态转化生成α-FeOOH;另一方面,大部分γ-FeOOH可与Fe2+结合生成Fe3O4/γ-Fe2O3,具体过程如下[7]:
在裸钢状态时,阴极首先发生O2的还原反应,从而平衡Fe的溶解:
根据O2扩散至双电层界面的受限程度,阴极过程还会包括不同程度的析氢反应:
随着暴露时间的延长,碳钢表面有锈层的存在,阴极反应发生一定的变化,由于γ-FeOOH是不稳定的产物,具有很强的还原性,会增加阴极反应的活性区域,Fe的溶解不会立即通过与O2的反应来平衡,而是通过还原已存在的锈来平衡[33]:
由机械-化学效应[34,35]可知,弹性拉应力会显著提高Q235钢基体的电化学反应活性,促进腐蚀初期钢基体的阳极溶解,即反应(8),从而生成更多的Fe2+。更多的Fe2+促进了反应(9)和(10),继而促进反应(11)和(12)中γ-FeOOH的生成。由于反应(9)~(12)均在液相电解质中进行,γ-FeOOH的生成属于快步骤。相较于γ-FeOOH的生成,反应(13)中γ-FeOOH向α-FeOOH的转变先后经历了溶解析出和固态转变,反应(14)中γ-FeOOH向Fe3O4的转变在固-液界面进行,因而反应(13)和(14)均属于慢步骤。由此可知,钢基体所受弹性拉应力促进了γ-FeOOH的生成,但更多的γ-FeOOH无法及时通过反应(13)和(14)转变为α-FeOOH和Fe3O4,因而导致图6b中所示结果:随钢基体所受弹性拉应力水平增加,锈层中γ-FeOOH的质量分数增加,而α-FeOOH和Fe3O4的质量分数减小。而且,由于γ-FeOOH的结构致密性在该3种物相中最差,因而锈层的致密性随着钢基体所受弹性拉应力水平增加而降低。锈层的致密性降低会加速O2在锈层中的扩散(图11b),促进反应(10)~(12)发生,从而进一步促进锈层中γ-FeOOH的生成和累积。
3.2 弹性拉应力对锈层结构的影响
由上述讨论可知,Q235碳钢基体所受弹性拉应力通过促进锈层形成过程中γ-FeOOH的生成,从而最终提高了锈层中γ-FeOOH的质量分数。这一成分的变化可能是导致锈层致密性降低的主要原因。在Q235碳钢锈层的主要物相成分中,γ-FeOOH是最先生成的产物,结晶完成度较高(图6a),但结晶后的结构疏松多孔,致密性较差[36];Fe3O4/γ-Fe2O3同样有着较高的结晶完成度(图6a),且有比较好的致密性和稳定性[4];而α-FeOOH由无定形的FeO x (OH)3 - 2x 转变而来,其结晶完成度较低,无定形态α-FeOOH和结晶态α-FeOOH结合,形成了锈层中致密性最高且最稳定的相[37]。因此,诸多研究[38~41]采用α-FeOOH(或α-FeOOH + Fe3O4/γ-Fe2O3)与γ-FeOOH的质量比(即r2 / r1或(r2 + r3) / r1)来表示锈层的保护性。实际上,r2 / r1或(r2 + r3) / r1的值也体现了其与锈层致密性的正相关性。图12对比展示了r2 / r1和(r2 + r3) / r1的值与锈层密度随钢基体所受弹性拉应力水平的变化趋势。可见,r2 / r1、(r2 + r3) / r1和锈层密度均与应力水平呈良好的线性关系,且锈层密度的斜率(-1.4244)介于r2 / r1和(r2 + r3) / r1 2者的斜率(-0.9465和-2.5597)之间。因此,可以认为锈层的致密性与锈层中物相成分存在直接的相关性,基体所受弹性拉应力降低了锈层中α-FeOOH (或α-FeOOH + Fe3O4/γ-Fe2O3)与γ-FeOOH的质量比,从而导致了锈层致密性的降低。
图12
图12
锈层中r2 / r1、(r2 + r3) / r1和锈层密度随钢基体所受弹性拉应力水平的变化
Fig.12
Variations of r2 / r1 and (r2 + r3) / r1 in the rust layer and the rust layer density with elastic tensile stress level on the steel (r1—mass fraction of γ-FeOOH, r2—mass fraction of α-FeOOH, r3—mass fraction of Fe3O4/γ-Fe2O3)
总体来说,锈层的保护性与其厚度和致密性均密切相关。其中,锈层的保护性与其厚度的关系应是简单的正相关:锈层的厚度越大,离子电迁移通过整个锈层的阻力越大(Rrust越大),因而锈层的保护性越好。然而,锈层的保护性与锈层致密性的关系并非简单的单调相关:一方面,锈层致密性的增加有利于提高锈层对基体整体的隔离性能,腐蚀性介质与基体接触的难度加大,从而抑制阳极溶解反应,减缓腐蚀的发生;另一方面,锈层致密性的增加可能导致锈层内外的物质交换困难,增加锈层内侧微环境的闭塞性,从而促进锈层内侧局部微环境的酸化和离子浓度升高,加速腐蚀的发生[20]。在本工作中,Q235碳钢锈层的致密性随基体所受应力水平提高而降低。这一结果体现在电化学过程上表现为A加载方式下W随应力水平提高而减小(图11b)。结合图11a所示A和B 2种加载方式下的Rct可知,加载方式B下(弹性拉应力未参与锈层的形成过程)锈层内侧微环境的闭塞性较A加载方式下更强。因此,基体所受弹性拉应力对Q235碳钢锈层致密性的降低作用减弱了锈层内侧微环境的闭塞性,反而有利于减缓腐蚀的发生。综上所述,Q235碳钢在弹性拉应力作用下形成的锈层,其保护性的提高来自于2方面:一是弹性拉应力促进锈层厚度增加,二是弹性拉应力通过降低锈层致密性减弱锈层内侧微环境的闭塞性。
4 结论
(1) 弹性拉应力通过促进Q235碳钢基体阳极溶解,从而促进作为前驱体的γ-FeOOH物相的生成。由于涉及固态转变或在固-液界面进行,γ-FeOOH向α-FeOOH和Fe3O4/γ-Fe2O3的转变速率较γ-FeOOH的生成速率慢。因此,随基体所受弹性拉应力水平的增加,锈层中γ-FeOOH的质量分数增加,而α-FeOOH和Fe3O4的质量分数减小。
(2) Q235碳钢锈层的致密性与锈层中物相成分直接相关,基体所受弹性拉应力通过降低锈层中α-FeOOH (或α-FeOOH + Fe3O4/γ-Fe2O3)与γ-FeOOH的质量比,导致锈层致密性降低。
(3) 基体弹性拉应力一方面促进了锈层厚度增加,提高锈层对基体整体的隔离性能;另一方面通过降低锈层致密性减弱锈层内侧局部环境的闭塞性。这2方面作用导致碳钢在受弹性拉应力作用下形成锈层的保护性高于在无弹性拉应力下形成的锈层。
参考文献
Effect of temperature on corrosion behavior of low-alloy steel exposed to a simulated marine atmospheric environment
[J].
Evolution of the rust layers formed on carbon and weathering steels in environment containing chloride ions
[J].
Corrosion behavior of 316L stainless steels exposed to salt lake atmosphere of Western China for 8 years
[J].
Marine atmospheric corrosion of carbon steel: A review
[J].
Influence of outer rust layers on corrosion of carbon steel and weathering steel during wet-dry cycles
[J].
Electrochemical reduction of ferric corrosion products and evaluation of galvanic coupling with iron
[J].
Evolution of initial atmospheric corrosion of carbon steel in an industrial atmosphere
[J].
Atmospheric corrosion of carbon steel and weathering steel in three environments
[J].
碳钢、耐候钢在3种典型大气环境中的腐蚀规律研究
[J].
Study of corrosion evolution of carbon steel exposed to an industrial atmosphere
[J].
Effect of prior plastic deformation and deformation rate on the corrosion resistance of AISI 321 austenitic stainless steel
[J].
Effect of Cl- concentration on the SCC behavior of 13Cr stainless steel in high-pressure CO2 environment
[J].
Electrochemical characterization and stress corrosion cracking of E690 high strength steel in wet-dry cyclic marine environments
[J].
Effect of plastic deformation on the corrosion resistance of ferritic stainless steel as a bipolar plate for polymer electrolyte membrane fuel cells
[J].
Numerical study on hydrogen permeation of ferritic steel evaluated under constant load
[J].
Corrosion behaviour of low-carbon bainitic steel under a constant elastic load
[J].
Elastic stress impacting on the rust layer of S450EW weathering steel through magnetomechanical effect
[J].
A study on the initial corrosion behavior of carbon steel exposed to a simulated coastal-industrial atmosphere
[J].Carbon steels as common structural material have been widely used for basic facilities with the development of the city. In these service environments, carbon steel would inevitably encounter the atmospheric corrosion. Especially, the corrosion of carbon steels exposed to coastal-industrial atmosphere is very outstanding. However, the initial corrosion mechanism of carbon steel subjected to coastal-industrial environment still need to be clarified, which would be vital for predicating the subsequent corrosion process. In addition, although many scholars studied the synergism of SO2 and Cl-, which obviously accelerates the corrosion of steel and reduces its service life, there is few research about the effect of the synergism of SO2 and Cl- (in different proportion) on the early corrosion behavior of the carbon steel. Therefore, it is of great importance to investigate the initial corrosion mechanism of carbon steel and the effect of the synergism of SO2 and Cl- (in different proportion) in the coastal-industrial atmosphere. In present work, the initial corrosion behavior of Q235 carbon steel exposed to a simulated coastal-industrial atmosphere has been studied by weight loss, XRD, SEM and electrochemical measurements. Also, the effect of the synergism of SO2 and Cl- (in different proportion) on the early corrosion behavior of Q235 car bon steel has been investigated. The results indicate that the initial corrosion behavior of carbon steel exposed to a simulated coastal-industrial atmosphere presented a transition from corrosion acceleration to deceleration, and the kinetics of accelerated corrosion process followed the empirical equation D=Atn. A double-layered corrosion product was formed on the surface of carbon steel after 24 h: the loose outer layer and relative dense inner layer; the synergistic effect between SO2 and Cl- accelerated the corrosion of carbon steel. However, the change in the ratio of SO2 and Cl- had no significant effect on the corrosion loss of carbon steel, and had not changed the composition of corrosion products formed on carbon steel surface. SO2 caused the corrosion morphology of carbon steel to tend to uniform corrosion.
碳钢在模拟海洋工业大气环境中初期腐蚀行为研究
[J].采用失重分析、X射线衍射分析、扫描电镜分析及电化学测试分析方法对Q235碳钢在模拟海洋工业大气环境中的初期腐蚀历程和机理开展深入研究,并着重探究了不同比例SO<sub>2</sub>和Cl<sup>-</sup>的协同效应对碳钢初期腐蚀行为机制的影响。结果表明,Q235碳钢在模拟海洋工业大气环境中的初期腐蚀呈现由加速过程向减速过程转化的特点,且加速过程的腐蚀动力学仍遵循幂函数规律D=At<sup>n</sup>;腐蚀24 h后,腐蚀产物呈现双层结构,即疏松的外层和相对致密的内层。SO<sub>2</sub>和Cl<sup>-</sup>的协同效应会加速碳钢的腐蚀,但二者比例的变化对碳钢腐蚀失重影响并不明显,也没有改变腐蚀产物成分,SO<sub>2</sub>促使碳钢腐蚀形态趋向于均匀腐蚀。
The initial corrosion behavior of carbon steel exposed to the coastal-industrial atmosphere in Hongyanhe
[J].
碳钢在红沿河海洋工业大气环境中的初期腐蚀行为
[J].
Effect of chloride ion on corrosion resistance of Ni-advanced weathering steel in simulated tropical marine atmosphere
[J].
RIR-measurement and use in quantitative XRD
[J].The Reference Intensity Ratio (RIR) is a general, instrument-independent constant for use in quantitative phase analysis by the X-ray powder diffraction internal standard method. When the reference standard is corundum, RIR is known as I/Ic; These constants are collected in the Powder Diffraction File (1987), can be calculated, and can be measured. Recommended methods for accurate measurement of RIR constants are presented, and methods of using these constants for quantitative analysis are discussed. The numerous, complex constants in Copeland and Bragg's method introduced to account for superimposed lines can be simply expressed in terms of RIR constants and relative intensities. This formalism also permits introduction of constraints and supplemental equations based on elemental analysis.
Synergy of Cu and Sb to enhance the resistance of 3%Ni weathering steel to marine atmospheric corrosion
[J].
Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces
[J].
Influences of pH value, temperature, chloride ions and sulfide ions on the corrosion behaviors of 316L stainless steel in the simulated cathodic environment of proton exchange membrane fuel cell
[J].
The electrochemical behaviour of stainless steel AISI 304 in alkaline solutions with different pH in the presence of chlorides
[J].
Numerical approach for atmospheric corrosion monitoring based on EIS of a weathering steel
[J].
Benefit of the corrosion product film formed on a new weathering steel containing 3% nickel under marine atmosphere in Maldives
[J].
Effect of nickel on ion-selective property of rust formed on low-alloying weathering steel
[J].
Corrosion resistance and mechanical properties of low-alloy steels under atmospheric conditions
[J].
In situ Raman spectroscopic identification of rust formation in Evans' droplet experiments
[J].
In situ observation of initial rust formation process on carbon steel under Na2SO4 and NaCl solution films with wet/dry cycles using synchrotron radiation X-rays
[J].
A study for corrosion behavior of a new-type weathering steel used in harsh marine environment
[J].
Weathering steels: From empirical development to scientific design. A review
[J].
The mechanochemical behavior of type 316L stainless steel
[J].
Effect of cathodic polarisation on stress corrosion cracking behaviour of a Ni(Fe, Al)-maraging steel in artificial seawater
[J].
Environmental conditions for akaganeite formation in marine atmosphere mild steel corrosion products and its characterization
[J].The corrosion of mild steel in chloride-rich atmospheres is a highly topical issue. The formation of the oxyhydroxide akaganeite (β-FeOOH) in this type of atmosphere leads to a notable acceleration of the steel corrosion process. The scientific literature contains many references to outdoor marine atmospheric tests, but so far has failed to clarify two basic matters in relation to akaganeite: the environmental conditions necessary for its formation, and its morphological characterization. Research has been performed at three atmospheric corrosion stations located at Cabo Vilano wind farm (Camariñas, Spain) at different distances from the shoreline (332, 590, and 2,400 m), with chloride deposition rates of 390, 74, and 29 mg/m2/day, respectively, with the exposure of mild steel specimens for 1 year. This paper reports the environmental conditions that generally led to the formation of akaganeite: an annual average relative humidity of around 80% or higher, and simultaneously, an annual average chloride deposition rate of approximately 60 mg/m2/day or higher. Rigorous characterization of akaganeite was performed by x-ray diffraction, scanning electron microscopy/energy dispersive spectroscopy, and transmission electron microscopy/selected area electron diffraction.
Initial corrosion behavior of carbon steel and weathering steel in Nansha marine atmosphere
[J].
碳钢和耐候钢在南沙海洋大气环境中的初期腐蚀行为
[J].
Advances in understanding atmospheric corrosion of iron. I. Rust characterisation of ancient ferrous artefacts exposed to indoor atmospheric corrosion
[J].
Recent progress in the study of protective rust-layer formation on weathering steel
[A].
Distinct beneficial effect of Sn on the corrosion resistance of Cr-Mo low alloy steel
[J].In this work, the beneficial effect of Sn addition on the corrosion resistance mechanism of Cr-Mo low alloy steel was studied. Results demonstrated that Sn improves the corrosion resistance of the steel matrix mainly by influencing the microstructural transformation. Sn addition and the synergistic effect of Sn, Cr, and Mo promote the formation of α-FeOOH, SnO2, SnO, Cr(OH)3, and molybdates, lead to the improved protection and stability of the rust layer. This synergistic effect also endows the inner rust layer with cation selectivity, preventing the further penetration of Cl- and inhibiting the localized corrosion of steel.