作为制造石油管道和天然气管道的主要材料,管线钢需要具有良好的力学性能、冲击韧性、抗腐蚀性能和焊接性能[1~4]。非金属夹杂物是影响管线钢性能的重要因素之一,例如钙铝酸盐夹杂物容易导致管线钢产生疲劳裂纹,且夹杂物尺寸越大、裂纹产生越早,严重缩短了管线钢的寿命[5~8],因此,必须严格控制管线钢中的非金属夹杂物。近几十年来,关于钢液中夹杂物的控制得到了广泛关注,钢液中夹杂物的控制技术已逐渐成熟,通过钢液脱氧可以控制夹杂物的种类和数量,通过加合金和调整精炼渣成分可以改性夹杂物,通过吹Ar搅拌或真空处理能够有效去除夹杂物等[9~14]。近年来,学者们[15~22]发现,在钢的凝固、冷却和保温过程,钢中夹杂物依然会发生转变,仅控制好钢液中的夹杂物是不够的。钢的加热时间、温度以及经历的冷却过程均会影响钢产品中夹杂物的成分。18Cr-8Ni不锈钢在1100℃下保温1 h后,钢中夹杂物从加热前的球形MnO-SiO2转变为加热后的MnO-Cr2O3尖晶石[17,18,20]。Yang等[19]通过实验和热力学计算对比了管线钢中间包钢液中夹杂物成分和连铸坯中夹杂物成分的差别。结果表明,中间包钢液中夹杂物主要为CaO-Al2O3夹杂物,而连铸坯中夹杂物则为MgO-CaO-Al2O3-CaS。管线钢连铸坯不同位置处夹杂物成分也存在明显差别,Ren等[23]的研究表明,在连铸坯表层夹杂物成分主要为CaO-Al2O3类夹杂物,靠近表层1/4距离处夹杂物中CaS含量最高、CaO含量最低,夹杂物成分产生这种差异的原因主要是连铸坯不同位置所经历的降温历程不同,即冷却速率不同,由此引起的不同位置处钢和夹杂物之间发生化学反应速率和平衡的不同。
为了明确冷却速率对降温过程钢中夹杂物成分转变的影响,本工作采用高温共聚焦显微镜(CSLM)研究了不同冷却速率下管线钢中夹杂物成分的变化,并计算分析了温度对管线钢中夹杂物的热力学平衡成分的影响,最后基于钢-夹杂物之间的热力学平衡建立了管线钢凝固和冷却过程中夹杂物成分转变的动力学模型,定量分析了冷却速率对夹杂物成分转变的影响。
1 实验方法
实验材料为实际工业生产的管线钢连铸坯,连铸坯断面尺寸为1450 mm × 230 mm,研究试样所取位置为连铸坯宽度方向中心位置,距离内弧表面1/4处。其化学成分(质量分数,%)为:C 0.071,Si 0.19,Mn 1.67,T.Al 0.045,T.Mg 0.0002,,T.S 0.0005,T.Ca 0.0009,T.O 0.002,Fe余量(其中,T.为Total,代表总量)。将坯料加工成直径7 mm、高4 mm的圆饼试样,利用砂轮机将试样底面和侧面打磨光滑以除去表面的铁锈,顶面进行磨抛,随后利用VL2000DX-SVF18SP型CSLM严格控制升降温速率,进行试样的升温、保温和冷却实验。实验中选取未进行过升温、保温和冷却过程的试样作为空白试样。
CSLM设备密封性能佳,能够精准控制试样温度的变化,炉身上部连接了抽真空装置与吹Ar气装置,为高温实验提供了稳定的惰性气氛。加热炉的冷却系统由水冷循环系统和氦气急冷系统构成,最大升温速率可达300℃/s,最大冷却速率可达100℃/s[24]。根据文献[25]可知,在连铸过程中,连铸坯靠近内弧侧1/4位置处冷却速率长时间保持在600℃/min左右,所以本工作将最大冷却速率定为800℃/min。将试样先以50℃/min的速率升温到200℃,然后以300℃/min的速率升温到1600℃,保温5 min,随后分别以800、600、400、200、100和5℃/min的速率降温到室温,实验过程如图1所示。待试样冷却后,对其进行磨平和抛光,使用配备有X射线能谱仪(EDS)的Aspex自动扫描电子显微镜(SEM)分析钢中非金属夹杂物的数量、直径和成分。SEM加速电压为15 keV,放大倍数1000倍,每个试样检测10 mm2以上,扫描最小夹杂物直径为1 μm。
图1
图1
试样的升降温过程示意图
Fig.1
Schematic of the temperature rise and drop processes of samples
2 实验结果
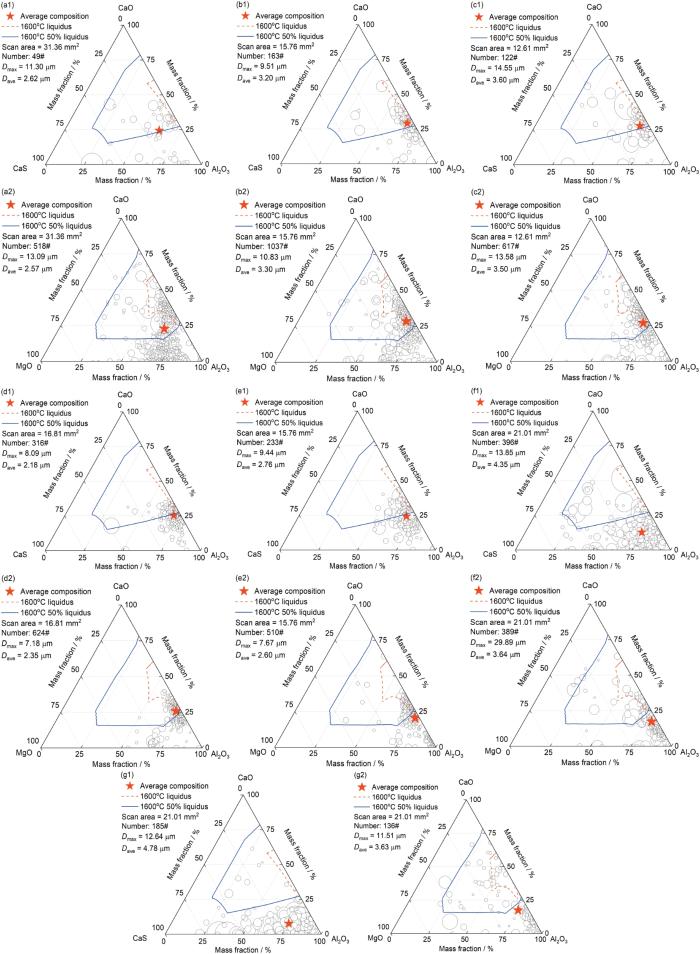
图2为不同冷却速率下管线钢中非金属夹杂物CaO-Al2O3-MgO-CaS四元成分的分布,采用CaO-Al2O3-CaS和CaO-Al2O3-MgO 2个三元成分表征夹杂物的成分,当夹杂物中CaS含量大于MgO含量时,将夹杂物投影到CaO-Al2O3-CaS三元成分图,反之则投影到CaO-Al2O3-MgO三元成分图中,图中每个圆圈代表一个夹杂物,圆圈的大小代表夹杂物的直径。在加热前,夹杂物成分主要为Al2O3-CaO-MgO,主要分布在CaO-Al2O3一侧,少量夹杂物中MgO的含量较高,夹杂物中的CaS含量很低,绝大多数夹杂物中CaS含量低于2.37% (质量分数,下同) (图2a1和a2)。当冷却速率为800℃/min时,试样在2 min内由1600℃冷却到室温,夹杂物和钢液的反应时间非常短,夹杂物成分没有明显变化,接近于加热前的成分(图2b1和b2)。当冷却速率减小到600℃/min时,夹杂物主要分布在CaO-Al2O3一侧,但相较于冷却速率为800℃/min的情况,夹杂物中CaS含量有所增加(图2c1和c2)。随着冷却速率进一步降低到400℃/min,夹杂物平均直径逐渐变小,CaS含量进一步增加(图2d1和d2)。当冷却速率为200℃/min时,夹杂物由CaO-Al2O3一侧缓慢向Al2O3-CaS一侧移动(图2e1和e2)。当冷却速率进一步降低为100℃/min时,夹杂物中CaS含量明显增加,夹杂物进一步向Al2O3-CaS侧移动(图2f1和f2)。当冷却速率降低到5℃/min时,CaS含量大于MgO含量的夹杂物数量显著升高,夹杂物成分主要为Al2O3-CaO-CaS,其中,CaS含量达到10.55% (图2g1和g2)。
图2
图2
不同冷却速率下管线钢中夹杂物的成分分布
Fig.2
Compositions of inclusions at CaO-Al2O3-CaS (a1-g1) and CaO-Al2O3-MgO (a2-g2) ternary composition diagrams in the blank sample (a1, a2), and pipeline steels at cooling rates of 800oC/min (b1, b2), 600oC/min (c1, c2), 400oC/min (d1, d2), 200oC/min (e1, e2), 100oC/min (f1, f2), and 5oC/min (g1, g2) (Dmax—maximum diameter of inclusions, Dave—average diameter of inclusions. When CaS% > MgO%, inclusions were projected into CaO-Al2O3-CaS ternary composition diagram, otherwise inclusions were projected into CaO-Al2O3-MgO ternary composition diagram. Each circle in the figure represents an inclusion, and the size of the circle represents the diameter of the inclusion)
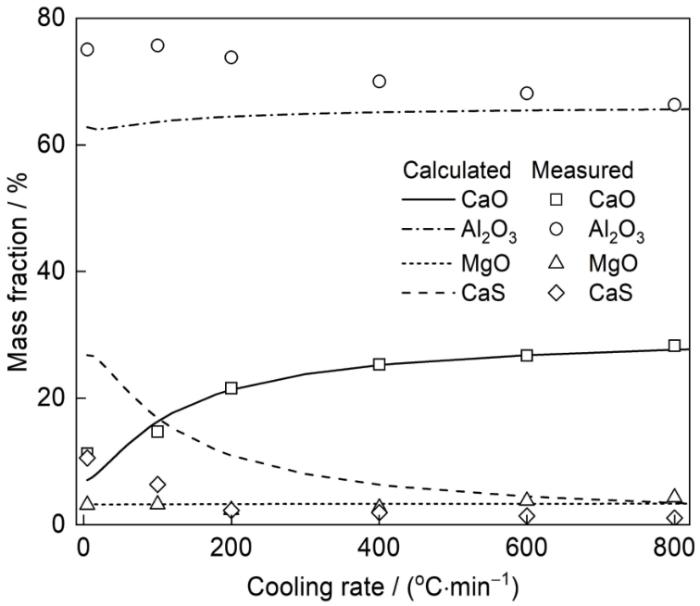
图3为不同冷却速率下夹杂物平均成分的变化。由图可见,当冷却速率大于200℃/min时,冷却速率对室温下夹杂物成分的影响较小;当冷却速率小于200℃/min时,随冷却速率的降低,室温下夹杂物成分发生了显著的变化,主要体现在夹杂物中CaO和CaS含量上,冷却速率越小,室温下夹杂物中CaO含量越低,CaS含量越高。冷却速率为200、100和5℃/min时,夹杂物中CaO含量依次为21.57%、14.72%和11.24%,CaS含量依次为2.27%、6.36%和10.55%。随冷却速率由800℃/min降低到5℃/min,夹杂物中Al2O3含量由66.33%上升至75.06%,MgO含量由4.33%降至3.15%。
图3
图3
不同冷却速率下夹杂物平均成分的变化
Fig.3
Variations of average composition of inclusions at different cooling rates
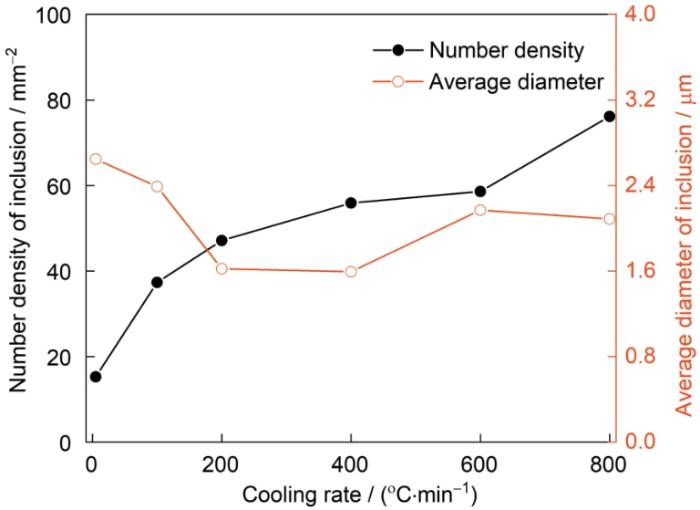
图4为不同冷却速率下夹杂物数密度和平均直径的变化。冷却过程钢中夹杂物数量和直径的变化主要取决于夹杂物的上浮去除和固体钢中夹杂物与钢基体反应的影响。随着冷却速率的降低,夹杂物数密度明显减小,由76.15 mm-2降至15.28 mm-2,主要原因在于夹杂物密度小于钢液密度,夹杂物会逐渐由钢液内部上浮到钢液表面,被从钢液内部去除,随着冷却速率的降低,钢液凝固所需的时间变长,因此这一去除过程持续的时间也越长,从而使得夹杂物数密度降低。对于夹杂物平均直径而言,冷却速率为200和400℃/min条件下夹杂物平均直径最低。冷却速率大于等于400℃/min时,虽然钢液凝固所需时间极短,但依然存在夹杂物在钢液中的上浮去除,是影响夹杂物平均直径的主要因素;直径越大的夹杂物上浮去除越明显,故夹杂物的平均直径随冷却速率降低而有所减少。但是,当冷却速率小于等于200℃/min时,在固体钢中,钢基体和夹杂物发生明显的反应,部分CaO夹杂物转变为CaS,夹杂物的直径有所增大,主要是因为CaO密度大于CaS密度,且CaO夹杂物转变为CaS夹杂物后质量增大,因此转变后夹杂物体积增大,直径增大。而且钢-夹杂物反应的影响大于钢液中夹杂物上浮的影响,因此,夹杂物平均直径随着冷却速率的降低而增大。
图4
图4
不同冷却速率下夹杂物数密度和平均直径的变化
Fig.4
Variations of the number density and average diameter of inclusions at different cooling rates
图5
图5
不同冷却速率下不同直径夹杂物的数密度分布
Fig.5
Distributions of inclusion number density with diameter at cooling rates of 800oC/min (a), 600oC/min (b), 400oC/min (c), 200oC/min (d), 100oC/min (e), and 5oC/min (f)
3 分析讨论
3.1 冷却过程钢中夹杂物成分演变的热力学分析
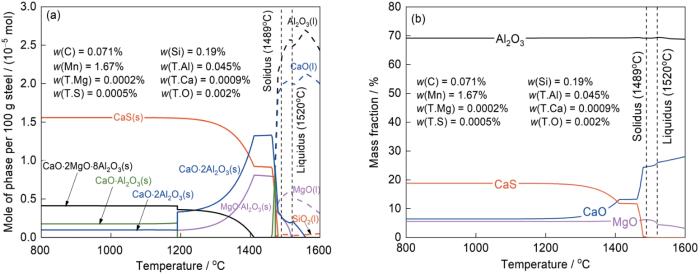
在冷却过程中,夹杂物和钢基体之间存在化学反应,随着温度的变化,钢和夹杂物之间的反应平衡也发生变化。为了分析钢和夹杂物之间的反应平衡,利用FactSage7.1热力学计算软件计算了800~1600℃范围内钢中夹杂物的热力学平衡成分,计算过程选用FactPS、FToxid、FSstel数据库,计算结果如图6所示。图6a为钢液在温度降低的过程中物相的转变。当温度高于1520℃时,钢为液态,钢中夹杂物主要为液态CaO-Al2O3-MgO和少量液态SiO2相。随着温度的降低,固相夹杂物逐渐生成。当温度低于1550℃时,固相CaO·2Al2O3开始生成,生成量先增加后减少。当温度为1515℃时,MgO·Al2O3尖晶石相生成,含量变化趋势与固相CaO·2Al2O3一致。在温度低于1410℃时,固相CaO·2MgO·8Al2O3生成并逐渐增加,而固相MgO·Al2O3和CaO·2Al2O3逐渐减少。当温度低于1475℃,液相CaO基本消失,同时固相CaS生成,且快速增加,最后在温度低于1200℃时趋于稳定。图6b为将夹杂物相转换为简单氧化物或硫化物的结果。在温度为1600℃时,夹杂物成分为68.78%Al2O3-28.07%CaO-3.15%MgO。随着温度的降低,夹杂物中CaO含量逐渐减少,CaS和MgO含量逐渐增加,Al2O3含量几乎不变,最终夹杂物成分基本稳定在69.20%Al2O3-6.44%CaO-5.55%MgO-18.81%CaS。夹杂物由Al2O3-CaO(-MgO)转变为Al2O3-CaS-(CaO-MgO),与实验结果相吻合。在冷却过程中,夹杂物中CaO和CaS析出的竞争机制与温度有关,当温度高于1475℃时,CaS是热力学不稳定相,所以夹杂物中不含有CaS,当温度降低到低于1475℃时,CaS逐渐成为热力学优势相,夹杂物中发生CaO转变为CaS的反应,如
图6
图6
管线钢凝固冷却过程中物相的转变和夹杂物热力学平衡成分随温度的变化
Fig.6
Evolutions of phase (a) and thermodynamic composition of inclusions (b) in the pipeline steel over temperature during steel solidification and cooling (w(i)—mass fraction of element i, T.—total, l—liquid, s—solid)
3.2 冷却过程钢中夹杂物成分演变的动力学分析
管线钢中夹杂物初始成分主要为CaO-Al2O3-MgO以及极少量CaS的复合夹杂物。由图3结果可知,随着冷却速率的降低,夹杂物成分的变化逐渐明显,夹杂物中CaO含量明显降低,CaS含量显著升高。冷却速率较快时,夹杂物和钢基体之间没有充足的反应时间,因此夹杂物成分变化较小。而当冷却速率较慢时,钢和夹杂物处于高温下的时间较长,夹杂物与钢基体之间的反应较为充分,使夹杂物成分发生了显著的变化。
在管线钢凝固和冷却过程中,夹杂物成分发生转变需要同时具备热力学和动力学2个条件。在热力学方面,随着温度的逐渐降低,钢基体与夹杂物之间的化学平衡发生了转变;在动力学方面,冷却过程中夹杂物的转变速率主要受钢中元素扩散速率的影响。冷却速率越小,钢-夹杂物之间反应的时间就越长,夹杂物的最终成分就越接近平衡态成分。
为了进一步研究管线钢冷却过程中夹杂物成分的转变机理,本工作建立了管线钢与夹杂物之间反应的动力学模型并自主编程进行求解,用于预报冷却速率对钢中夹杂物成分演变的影响。假设钢和夹杂物均是均一相,不存在元素或成分的不均匀分布。模型中每一个时间步长内的计算主要分为2步。(1) 钢基体中的元素在边界层和钢基体之间由于浓度差进行传质,元素传质公式如
表1 Al、 Mg、Ca、S、O在液态、δ和γ钢中的扩散系数[26~28]
Table 1
| Element | In liquid | In δ | In γ |
|---|---|---|---|
| Al | 3.5 × 10-09 | 5.9 × exp(-241186 / (RT )) / 10000 | 5.15 × exp(-245800 / (RT )) / 10000 |
| Mg | 3.5 × 10-09 | 0.76 × exp(-224430 / (RT )) / 10000 | 0.055 × exp(-249366 / (RT )) / 10000 |
| Ca | 3.5 × 10-09 | 0.76 × exp(-224430 / (RT )) / 10000 | 0.055 × exp(-249366 / (RT )) / 10000 |
| S | 4.1 × 10-09 | 4.56 × exp(-214639 / (RT )) / 10000 | 2.4 × exp(-223426 / (RT )) / 10000 |
| O | 2.7 × 10-09 | 0.0371 × exp(-96349 / (RT )) / 10000 | 5.75 × exp(-168454 / (RT )) / 10000 |
图7为不同冷却速率下2 μm直径的夹杂物成分演变的计算值和实验值之间的对比,图中的点为实验值,线为计算值。由图可见,冷却速率对夹杂物成分的影响主要体现在夹杂物中CaO和CaS含量的差别。随着冷却速率由800℃/min降低到5℃/min,夹杂物中CaO含量由27.63%降至7.04%,CaS含量由3.44%增至26.77%。冷却速率对夹杂物中MgO和Al2O3含量变化的影响较小。模型计算结果较为准确地预报出了夹杂物中CaO含量的变化。
图7
图7
不同冷却速率下2 μm直径的夹杂物成分计算值和实验值的对比
Fig.7
Comparisons of calculated and measured values of inclusion composition with 2 μm diameter at different cooling rates
通过分析
图8
图8
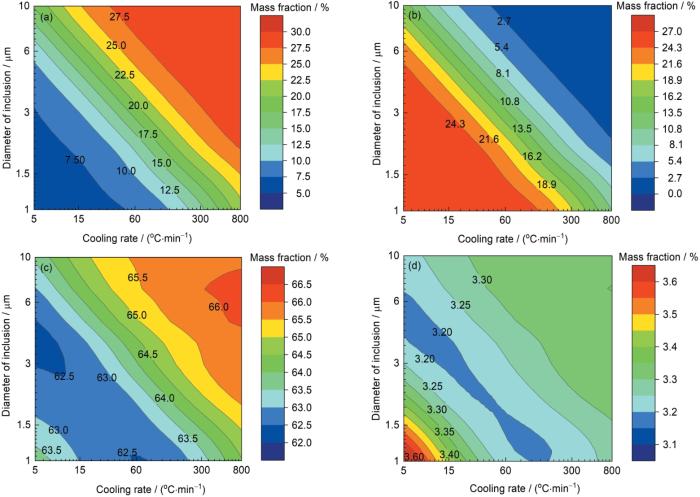
夹杂物成分随着冷却速率和夹杂物直径的变化
Fig.8
Variations of the composition of inclusions with the cooling rates and their diameters
(a) CaO (b) CaS (c) Al2O3 (d) MgO
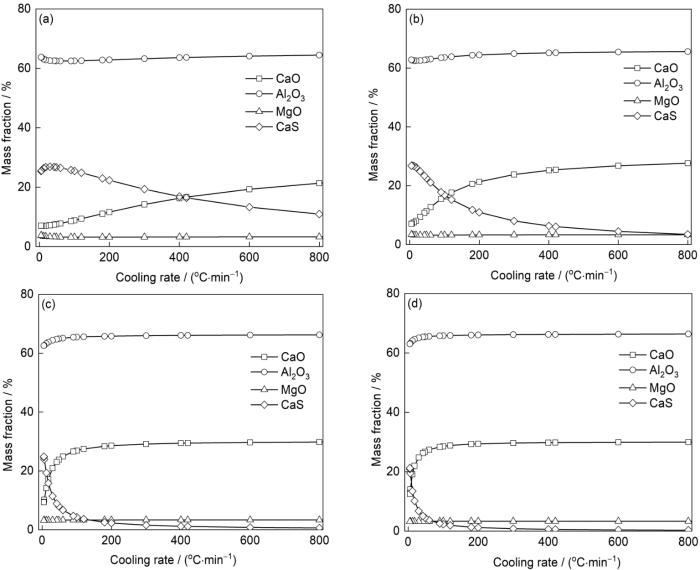
根据图8所示的夹杂物成分图,分别以纵坐标1、2、5、7 μm沿横轴取直线上的值,便可以得到不同直径的夹杂物成分随冷却速率的变化,如图9所示。夹杂物中MgO和Al2O3含量在所取夹杂物直径范围波动较小。当夹杂物直径为1 μm时,随着冷却速率的减低,夹杂物中CaS的含量逐渐升高并最终超过CaO的含量,CaS和CaO含量相等时的冷却速率约为400℃/min。而当夹杂物直径为2 μm时,夹杂物中CaO含量等于CaS含量时的冷却速率约为100℃/min。当夹杂物直径进一步增大为5或7 μm时,夹杂物中CaO和CaS含量产生交点时的冷却速率远小于1℃/min。这表明相较于直径≥ 5 μm的夹杂物,直径< 5 μm的夹杂物具有较大的比表面积,从而动力学条件更为良好,因此随着冷却速率的变化更为明显,并且随着夹杂物直径的增大,CaO转变为CaS所需的冷却速率急剧减小。
图9
图9
不同直径夹杂物成分随着冷却速率的变化
Fig.9
Variations of the composition of inclusions with diameters of 1 μm (a), 2 μm (b), 5 μm (c), and 7 μm (d) with cooling rates
4 结论
(1) 随着冷却速率由800℃/min降低到5℃/min,夹杂物中Al2O3含量由66.33%增至75.06%,CaS含量由1.07%增至10.55%,CaO含量由28.27%降至11.24%,MgO含量由4.33%降至3.15%。夹杂物数密度由76.15 mm-2降至15.28 mm-2,主要原因在于随着冷却速率的降低,夹杂物上浮去除时间延长。夹杂物平均直径先由2.09 μm缓慢降至1.62 μm,后由于钢和夹杂物之间发生化学反应,增至2.65 μm。
(2) 热力学计算表明,温度为1600℃时夹杂物的成分为68.78%Al2O3-28.07%CaO-3.15%MgO,随着温度的降低,夹杂物中CaO含量减少,CaS和MgO含量增加,Al2O3含量略有降低,最终夹杂物成分稳定在69.20%Al2O3-6.44%CaO-5.55%MgO-18.81%CaS。冷却过程主要发生夹杂物中CaO和钢中[S]之间的反应以及MgO·Al2O3的生成反应。
(3) 凝固冷却过程中夹杂物成分转变的动力学模型结果表明,冷却速率对夹杂物中MgO和Al2O3含量变化的影响较小。夹杂物直径和冷却速率均对夹杂物中CaO和CaS含量有显著影响,在钢的凝固冷却过程,夹杂物中CaS含量超过CaO含量的转折冷却速率与夹杂物直径存在直接关系,夹杂物直径为1和2 μm时,这一转折冷却速率分别为400和100℃/min,而当夹杂物直径大于5 μm时,这一转折冷却速率则远小于1℃/min。
参考文献
Impact fracture behavior of X80 pipeline steel
[J].Pipeline steels have been widely used in a long-distance transportation of large amounts of crude oil or natural gas under high pressure due to their high transportation efficiency, low energy loss and production cost. For high pressure gas transmission pipelines made from high-strength steels an important problem is to know their fracture behavior in a long running process. In this paper, fracture toughness of X80 pipeline steel was measured by V-notch Charpy impact test at -20 and\linebreak -60 ℃. OM, SEM, EDS and EBSD were used to analyze its fracture mechanism. Experimental results show that the maximum impact load is slightly influenced by temperature, but with the decrease of temperature, crack forming work, crack propagating work and energy absorbed by crack arresting decrease significantly. During fracture process, the intensive tensile stress in the sample would induce the deformation bands formed around the fracture surface and grains elongated along the direction vertical to the main crack, but original austenite grain boundaries are hardly deformed, it would cause stress concentration and then produce intergranular fracture. Generally, brittle second phase particles could act as crack sources under intensive internal stress. In the crack propagation process, grains near the main crack are deformed and elongated tempestuously, resulting in their breaking and new grains with high angle grain boundary (HAGB) formed. Therefore, the grain size decreases and the fraction of HAGB increases in the crack propagation region. Temperature has great influence on the plastic deformation behavior of pipeline steel during facture process. At -20 ℃, the tested steel has good plasticity and the grains are refined during deformation, but at -60 ℃, they are difficult to deform and refine. Consequently, the grain size near crack propagation zones in the sample impacted at -20 ℃ is much smaller than the original grain size, but in -60 ℃ impacted sample the grain sizes has little difference.
X80管线钢的冲击断裂行为
[J].
Research on non-metallic inclusion control of quality pipeline steel
[J].
优质管线钢非金属夹杂物控制研究
[J].
Research on the non-metallic inclusions in X70 pipeline cast slab
[J].
X70管线钢铸坯中非金属夹杂物的研究
[J].
Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process
[J].The formation and evolution of nonmetallic inclusions in pipeline steel were investigated by SEM, EDS and INCA Feature Analysis System, with the industrial process of electric arc furnace → ladle furnace (LF) refining → vacuum degassing → continuous casting. The composition, size and amount of inclusions during refining process were discussed systematically. The results show that inclusions at each refining step are mainly small-particle inclusions (below 5 µm), and the total number of inclusions has been reduced significantly due to the refining effect of slag during LF refining. The calcium (Ca) treatment increases the amount of small inclusions. The types of inclusion are mainly Al2O3 and MnO–SiO2–Al2O3 before LF, and they are transformed into CaO–Al2O3, MgO–Al2O3 and CaO–MgO–Al2O3 during LF process. After Ca treatment, inclusions are changed to CaO–Al2O3–(CaS) and CaO–MgO–Al2O3–(CaS). Typical inclusions are still mainly CaO–Al2O3 and CaO–MgO–Al2O3 in tundish, but the composition of those inclusions has been changed and located to the low melting point region in ternary phase diagram. Such inclusions will further be removed as continuous casting approaches.
Characterization of inclusions of X80 pipeline steel and its correlation with hydrogen-induced cracking
[J].
Control of stringer shaped non-metallic inclusions of CaO-Al2O3 system in API X80 linepipe steel plates
[J].
Effect of the nonmetallic inclusions on the HIC behavior of X120 pipeline steel
[J].
夹杂物对X120管线钢氢致开裂的影响
[J].
Evolution of MnS inclusions in Ti-bearing X80 pipeline steel
[J].
Control of formation of spinel inclusion in type 304 stainless steel by slag composition
[J].
SUS304ステンレス鋼中スピネル介在物生成のスラグ組成による制御
[J].
Effect of slag composition on the concentration of Al2O3 in the inclusions in Si-Mn-killed steel
[J].
In situ observation of calcium aluminate inclusions dissolution into steelmaking slag
[J].
Effect of the CaO-Al2O3-based top slag on the cleanliness of stainless steel during secondary metallurgy
[J].
Transient inclusion evolution during modification of alumina inclusions by calcium in liquid steel: Part I. Background, experimental techniques and analysis methods
[J].
Transient inclusion evolution during modification of alumina inclusions by calcium in liquid steel: Part II. Results and discussion
[J].
Transformation of inclusions in a complicated-deoxidized heavy rail steels during heating
[J].
Transformation of inclusions in solid GCr15 bearing steels during heat treatment
[J].Laboratory heating experiments with a varied holding time of GCr15 bearing steels at 1498 K were performed to study the transformation of inclusions in solid GCr15 bearing steels during high temperature diffusion processes. Heating experiments at 1573 and 1648 K were also carried out to study the effect of these heating temperatures. Experimental results showed that inclusions transformed from Al2O3-CaO-(MgO) to Al2O3-CaS-(MgO-CaO) when the heat treatment was in the range of 1498 to 1648 K due to reactions between Al and S in the steel matrix and CaO in the inclusions. This is in good agreement with thermodynamic calculations. Moreover, the size of the inclusions hardly changed after heat treatment. The transformation rate of the inclusions depended strongly on both the heating temperature and the size of the inclusions. Kinetic analyses on the transformation of inclusions during heat treatment were performed based on a simplified analytical model. The mass transfer coefficients of CaO and CaS in inclusions were calculated, which ranged from 0.73 × 10−10 to 4.48 × 10−10 m/s.
Transformation of oxide inclusions in type 304 stainless steels during heat treatment
[J].
Changes of the nonmetallic inclusion by heating (Study on the nonmetallic inclusion in 18-8 stainless steel-II)
[J].
造塊·非金属介在物
[J].
Transformation of inclusions in pipeline steels during solidification and cooling
[J].
Effect of heat treatment on oxide inclusion in Si-killed 304 stainless steel
[J].
热处理对硅脱氧304不锈钢内氧化物夹杂的影响
[J].
Effect of cooling rate on oxide inclusions during solidification of 304 stainless steel
[J].
Transformation of inclusions in linepipe steels during heat treatment
[J].
Prediction of spatial distribution of the composition of inclusions on the entire cross section of a linepipe steel continuous casting slab
[J].
A review on dissolution behavior of non-metallic inclusions in-situ observed using high temperature confocal scanning laser microscope
[J].
高温共聚焦显微镜原位观察非金属夹杂物溶解行为研究进展
[J].
Prediction on the spatial distribution of the composition of inclusions in a heavy rail steel continuous casting bloom
[J].
Analysis of solute distribution in dendrites of carbon steel with δ/γ transformation during solidification
[J].
Simple model of microsegregation during solidification of steels
[J].