钢中添加的与O2亲和力强的元素先于基体Fe元素发生氧化,称为选择性氧化。根据氧化物在钢板上所处的位置,可将氧化分为外氧化和内氧化。外氧化是指扩散至表面的合金元素与O发生反应生成氧化物,内氧化是指O扩散至表面以下的次表层与基体中的金属元素反应生成氧化物。
当外氧化减少、内氧化增加时,带钢的可镀锌性问题可以得到改善。但是随着AHSS品种的不断丰富,高C、高合金成分的钢种逐渐增多,在利用提高退火气氛露点将Si、Mn外氧化转变成内氧化的同时,也带来了一些副作用。Wu等[21]及Zhang等[22]在研究水压对高强钢选择性氧化的影响时,观察到了钢板表面的脱碳,表面脱碳严重时会导致材料强度下降。Han等[23]研究发现内氧化对焊接电流区间有影响,内氧化会降低接触电阻和热输入,从而延迟熔核生长。Kalashami等[24]研究了内氧化对热镀锌双相(DP)钢的焊接液态金属脆性(liquid metal embrittlement,LME)的影响,发现晶界的内氧化会促使焊接时液态锌在晶界扩散。Kalashami等[25]指出,为了获得相同的熔核直径和焊点强度,存在内氧化的热镀锌钢板需要更高的焊接电流和更长的焊接时间。可见对于工业生产的热镀锌高强钢而言,内氧化并不是越厚越好,在使用内氧化技术时,需要找到能够兼顾多方面的合适的工艺窗口。
本工作以主成分为0.2%C-1.5%Si-2.5%Mn (质量分数)、目标抗拉强度为1180 MPa的热镀锌AHSS为研究对象,从实际应用出发,综合考虑了合金元素内外氧化和表面脱碳的控制,研究了连续退火气氛露点对Si、Mn选择性氧化和脱碳的影响,以期为AHSS表面微结构精确控制提供参考。
1 实验方法
实验用样板取自宝钢工业化生产的0.2%C-1.5%Si-2.5%Mn高强钢轧硬卷,试样尺寸为70 mm×120 mm,板厚为1.2 mm。使用NaOH浓度为2% (质量分数)的工业脱脂剂对轧硬板试样进行清洗处理,脱脂剂温度为40~60℃,脱脂后用流水冲洗干净,用压缩空气吹干。
模拟退火实验在Iwatani HDPS设备上进行,温度曲线如图1所示,包括升温、保温、冷却3个阶段。以5℃/s由室温加热至870℃后保温120 s,然后以-10℃/s冷却至70℃。升温和保温过程使用相同的5%H2-N2 (体积分数)退火气氛,露点(dew point)分别控制为-40、-20、0和+10℃;冷却过程使用100%N2,不控制露点。
图1
采用LECO 750A辉光放电发射光谱分析仪(glow discharge optical emission spectrometry,GD-OES)对退火样板和未退火的轧硬板进行表面元素深度分析,重点关注试样表层合金元素Si、Mn、C及O的深度分布。取Si、Mn元素深度分布曲线上靠近表面的峰值以及峰值一半位置的深度表征外氧化的程度;取C元素深度分布上5~6 μm位置C含量平均值表征钢板次表层的脱碳程度。
采用常规金相方法制备截面试样,使用EVO MA25扫描电镜(SEM)观察抛光状态的截面,观察钢板次表层的内氧化并测量内氧化层厚度;使用Axio Imager M2m显微镜(OM)观察4% (体积分数)硝酸酒精溶液侵蚀后的截面,观察钢板次表层的脱碳层并测量脱碳层的厚度;使用Future-Tech FM-7显微硬度计检测次表层显微硬度沿深度方向的分布。
选择露点为-40℃和+10℃ 2个典型试样,使用Helios Nanolab 600i双束聚焦离子束(FIB)显微镜制备截面透射电镜(TEM)样品,并在2010 F TEM下观察截面形貌。在FIB制样前,试样表面沉积Pt保护层,然后使用镓离子束轰击样品直到样品达到大约100 nm的厚度来完成TEM试样制备。使用扫描透射电子显微镜-能谱仪(STEM-EDS)进行成分分析,重点关注钢板表面的Si、Mn元素外氧化以及钢板次表层的Si、Mn元素内氧化。
2 实验结果
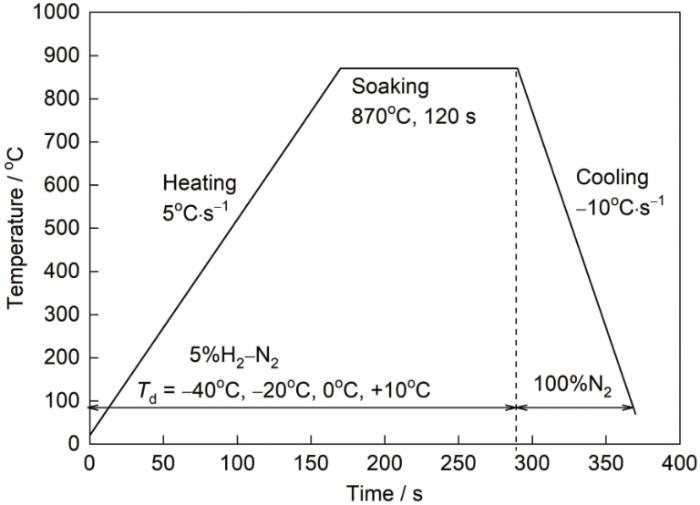
露点为-40℃模拟退火试样表面1000 nm深度范围内Fe、Mn、Si和O元素的深度分布曲线如图2所示,图中还标记了Mn、Si元素曲线上的峰值pMn和pSi,及Mn元素曲线上峰值一半位置的深度te (以此代表外氧化层的厚度)。在试样表层100 nm深度范围内,Mn和Si的含量均高于基体含量,而且Mn元素和O元素的曲线形状轮廓较接近,说明表面形成了较明显的Mn、Si元素外氧化。比较pMn和pSi可知,该试样Mn元素的外氧化比Si元素的外氧化更显著。图2中pMn和pSi相对应的深度分别为25.6和51.7 nm,te = 61 nm,比较上述深度和厚度位置的差异,推测外氧化层的成分可能存在沿厚度方向梯度分布的特征,靠近表面的氧化物Mn含量更高,而靠近基体的氧化物Si含量更高。
图2
图2
露点-40℃退火试样表面Fe、Mn、Si、O元素深度分布曲线
Fig.2
Fe, Mn, Si, and O depth profiles of the sample annealed at dew point of -40oC (pMn—peak value of Mn, pSi—peak value of Si, te—thickness of external oxidation)
图3
图3
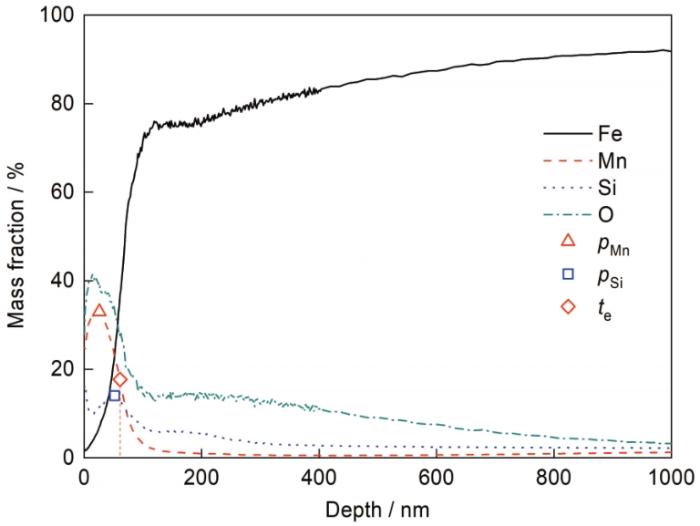
不同露点退火试样Mn、Si元素深度分布
Fig.3
Mn (a) and Si (b) depth profiles of the samples annealed at different dew points
图4
图4
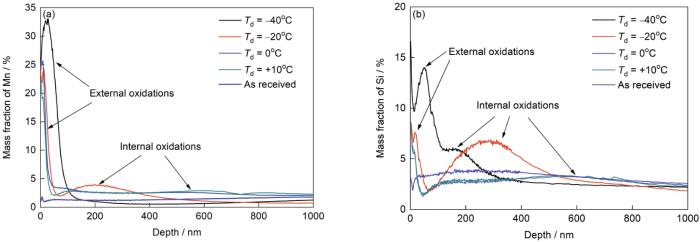
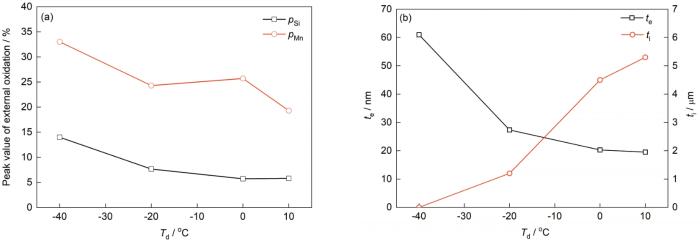
露点对Mn、Si内外氧化的影响
Fig.4
Effects of dew point on the external and internal oxidations of Mn and Si (ti—thickness of internal oxidation)
(a) pMn and pSi (b) te and ti
图5
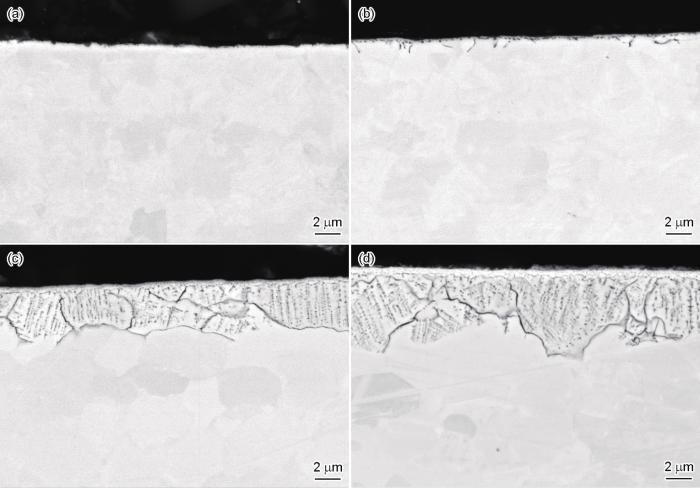
图5
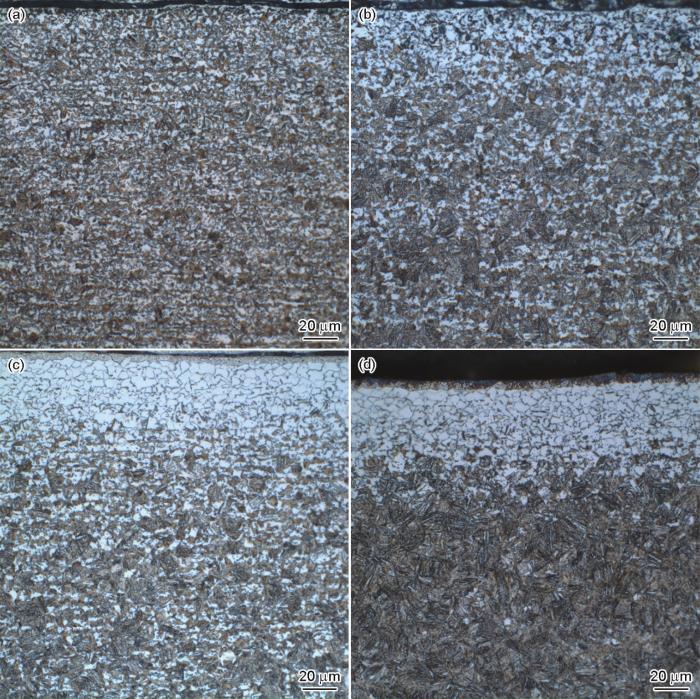
不同露点退火试样截面组织SEM像
Fig.5
Cross-sectional SEM images of the samples annealed at different dew points
(a) -40oC (b) -20oC (c) 0oC (d) +10oC
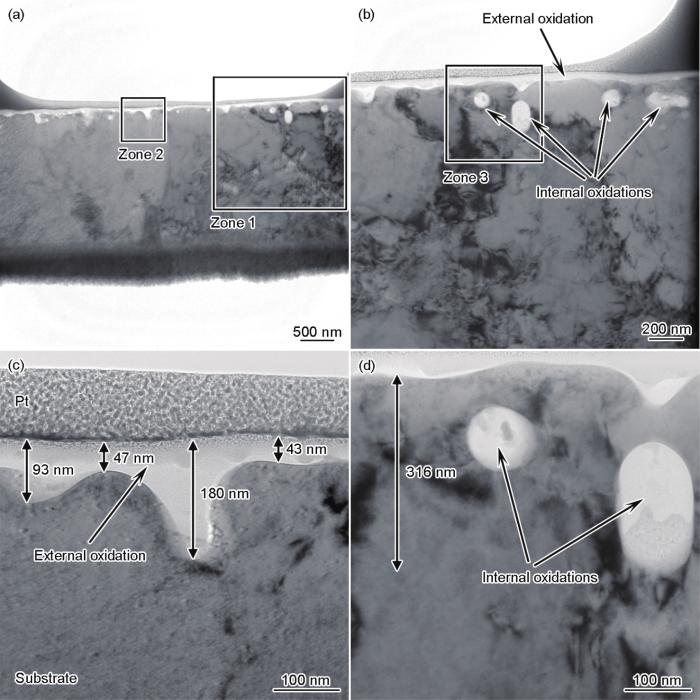
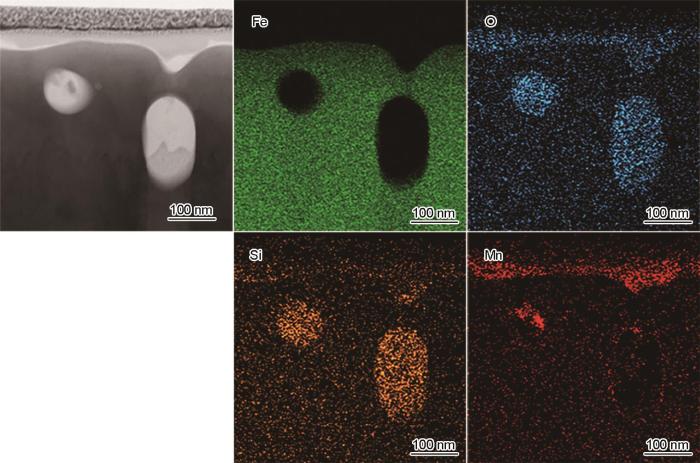
FIB制备的-40℃露点退火试样截面组织TEM像如图6所示,其中图6a为较低倍数的整体形貌,图6b~d为局部区域放大形貌。当露点为-40℃时,钢板表面有一层连续的外氧化层,微观上厚度并不均匀。若不考虑局部深入基体的位置,外氧化层的平均厚度为40~50 nm;若适当考虑局部深入基体的氧化,则外氧化层的平均厚度会略有增加,该结果与图4b显示的由GD-OES检测的外氧化厚度基本相当。除连续的外氧化外,在钢板次表层约300 nm的深度范围内,还观察到少量内氧化颗粒,如图6b和d中箭头所示。对照图3b中同一个试样相同深度内(100~300 nm)的Si元素含量,发现该位置的Si含量明显高于更深位置基体的Si含量,而相应位置的Mn含量仅为0.5%左右,明显低于基体Mn含量。局部元素面分布结果如图7所示,外氧化物中同时含有Mn和Si,其中Mn含量更高,而内氧化颗粒主要是Si的氧化物,仅在颗粒边缘检测到少量的Mn。由此可见,在露点为-40℃时,Si、Mn同时形成了连续致密的外氧化物,少量Si形成了内氧化颗粒。
图6
图6
聚焦离子束(FIB)制备的露点为-40℃退火试样截面组织TEM像
Fig.6
TEM image of the focused ion beam (FIB) prepared cross-sectional sample annealed at dew point of -40oC (a) and enlarged images of zone 1 (b), zone 2 (c), and zone 3 (d)
图7
图7
露点为-40℃退火试样TEM元素面分布结果
Fig.7
EDS elemental mapping of the TEM sample annealed at dew point of -40oC
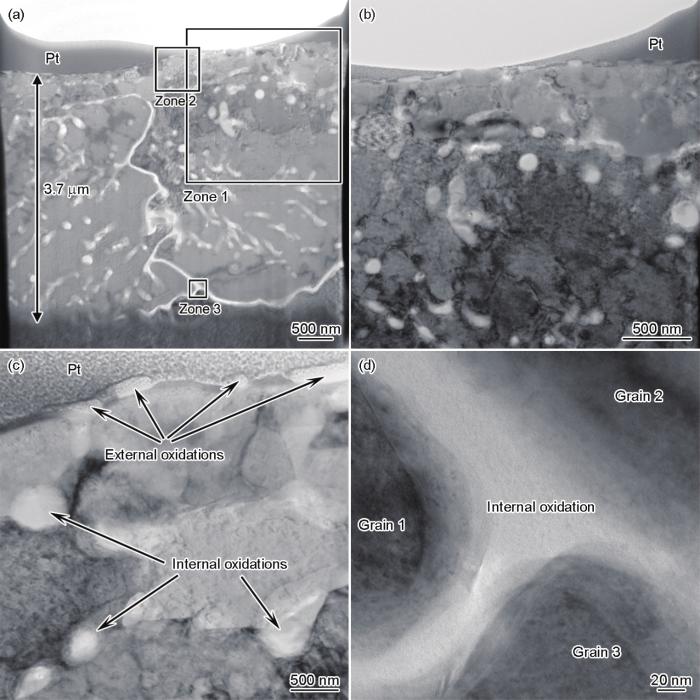
图8
图8
FIB制备的露点为+10℃退火试样截面组织TEM像
Fig.8
TEM image of the FIB prepared cross-sectional sample annealed at dew point of +10oC (a) and enlarged images of zone 1 (b), zone 2 (c), and zone 3 (d)
图9
图9
露点为+10℃退火试样上3个不同区域(见图8a)元素面分布
Fig.9
EDS elemental mapping of three different areas (in Fig.8a) on the sample annealed at dew point of +10oC
(a) upper zone (b) middle zone (c) bottom zone
图10
图10
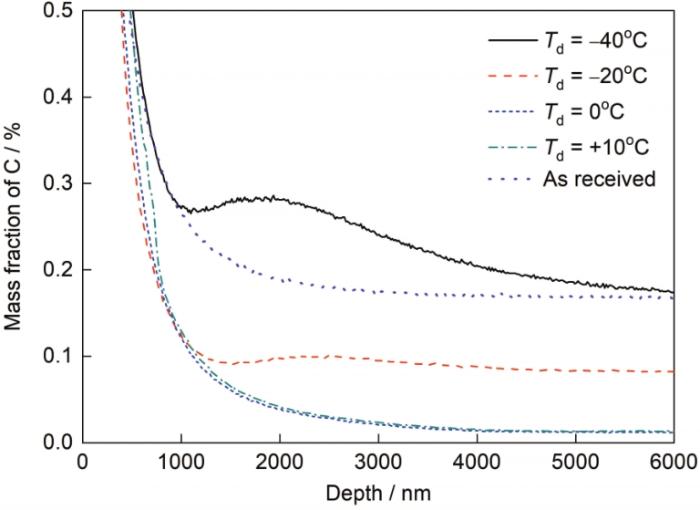
不同露点退火试样C元素深度分布
Fig.10
C depth profiles of the samples annealed at different dew points
图11
图11
露点对钢板次表层C含量及脱碳层厚度的影响
Fig.11
Effects of dew point on C content (a) and decar-burization thickness (b) in the subsurface area (td1—thickness of total decarburization, td2—thickness of full decarburization)
图12
图12
不同露点退火试样截面显微组织的OM像
Fig.12
Cross-sectional OM images of the samples annealed at different dew points
(a) -40oC (b) -20oC (c) 0oC (d) +10oC
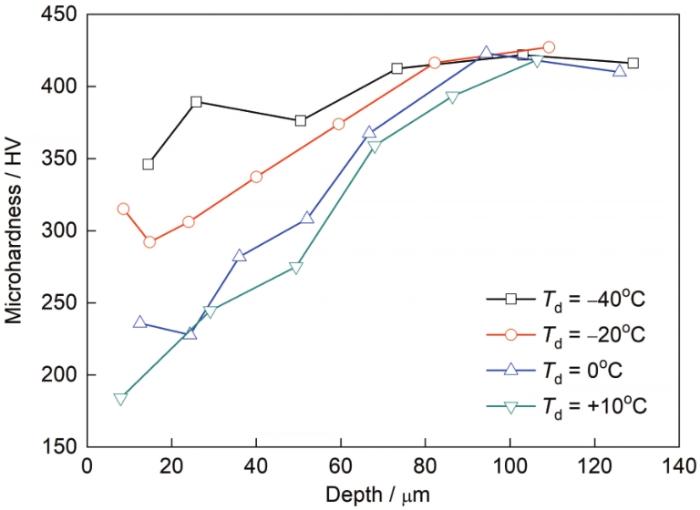
试样截面次表层显微硬度沿深度方向的变化如图13所示,因提高露点后钢板次表层出现了脱碳,因此次表层的显微硬度均降低。深度100 μm后基体的显微硬度超过了400 HV,当露点为-40℃时,仅最表层的显微硬度降至350 HV左右,其余位置和基体显微硬度差别不大。当露点为-20℃时,次表层显微硬度最低降至290 HV左右;当露点为0和+10℃时,次表层显微硬度最低降至200 HV左右。
图13
图13
露点对钢板次表层深度方向显微硬度的影响
Fig.13
Effects of dew point on microhardness along the depth in the subsurface area
3 分析与讨论
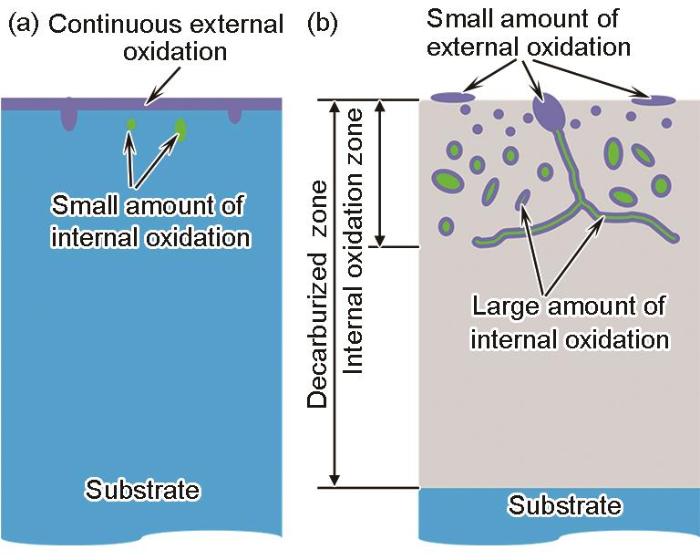
以上实验结果表明,当0.2%C-1.5%Si-2.5%Mn钢板在退火温度为870℃、保温时间为120 s、退火气氛为5%H2-N2的条件下进行连续退火时,退火气氛露点对Si、Mn内外氧化及表层脱碳的影响显著。低露点(-40℃)和高露点(+10℃)退火后的钢板表层示意图如图14所示。
图14
图14
露点对连续退火0.2C-1.5Si-2.5Mn钢选择性氧化及次表层脱碳的影响示意图
Fig.14
Schematic diagram showing the effect of dew point on selective oxidation and decarburization of 0.2C-1.5Si-2.5Mn high strength steel sheet during continuous annealing at the dew points of -40oC (a) and +10oC (b)
当露点为-40℃时,钢板表面会形成连续覆盖的Si-Mn外氧化膜,次表层形成少量Si的内氧化颗粒,钢板次表层未形成脱碳。当露点为+10℃时,钢板表面形成少量不连续的Si-Mn外氧化颗粒,在次表层约5 μm深度范围形成显著的内氧化层,在次表层约40 μm深度范围内形成脱碳。内氧化层中的氧化物可分成3类:第一类是靠近表面(深度为0~0.5 μm)的内氧化颗粒,其颗粒尺寸相对较小,主要是Si-Mn复合氧化物;第二类是位于深度大于0.5 μm的晶粒内部的内氧化颗粒,这些颗粒芯部是SiO2,外层为Si-Mn复合氧化物;第三类是位于晶界的网状内氧化物,同样是芯部为SiO2,外层为Si-Mn复合氧化物。
内氧化形成过程可以分为2步,第一步是O2和O原子溶于合金中,第二步是溶解的O原子和钢中的合金元素发生反应生成氧化物,内氧化深度(ξ)的表达式为[26]:
式中,
式中,
表1 根据露点计算的氧分压
Table 1
| Td / oC | T / oC | |||
|---|---|---|---|---|
| -40 | 870 | 0.05 | 1.27 × 10-4 | 8.08 × 10-23 |
| -20 | 870 | 0.05 | 1.02 × 10-3 | 5.20 × 10-21 |
| 0 | 870 | 0.05 | 6.03 × 10-3 | 1.82 × 10-19 |
| +10 | 870 | 0.05 | 1.21 × 10-2 | 7.37 × 10-19 |
Note:T—temperature,
表2 O、Mn、Si、C在钢中的扩散系数
Table 2
| Element | Temperature | Diffusion coefficient |
|---|---|---|
| oC | μm2·s-1 | |
| O | 870 | 1.507 × 101 |
| Mn | 870 | 4.227 × 10-3 |
| Si | 870 | 6.615 × 10-3 |
| C | 870 | 2.551 × 101 |
式中,
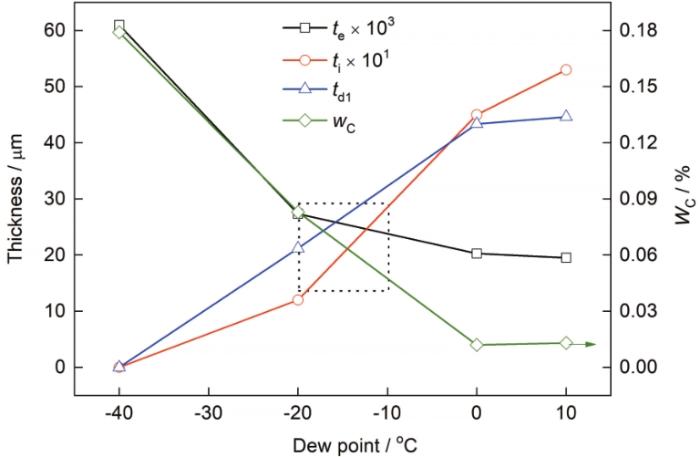
从露点对te、ti、td1以及wC的影响趋势来看,存在一个可以兼顾外氧化少且脱碳程度轻的区间。如图15所示,各曲线的交点区域可认为是兼顾外氧化和脱碳的区域,此时退火气氛露点位于-20~-10℃之间。
图15
图15
露点对te、ti、td1和wC的影响
Fig.15
Effect of dew point on te,ti, td1, and wC (wC—C content of surface area)
4 结论
(1) 提高连续退火加热段和均热段的气氛露点,可以促使0.2%C-1.5%Si-2.5%先进高强钢中的Si、Mn外氧化转变成内氧化,同时会引起钢板次表层发生明显的脱碳,形成次表层铁素体层,导致钢板表层硬度降低。
(2) 当露点提高到临界值后,继续提高露点对进一步减少外氧化的作用有限,但是内氧化层的厚度和脱碳层的厚度会继续显著增加,因此需要选择合适的露点范围,兼顾外氧化和脱碳层控制。
(3) 在退火温度为870℃、保温120 s、退火气氛为5%H2-N2的工艺条件下,为获得Si、Mn外氧化显著降低,同时内氧化层厚度< 1 μm、次表层仅出现部分脱碳的表面状态,合适的露点为-20~-10℃。
参考文献
Driving force and logic of development of advanced high strength steels for automotive applications
[J].
Third generation of advanced high strength sheet steels for the automotive sector: A literature review
[J].
Third generation 980 class AHSS: A viable alternative to replace press-hardenable steels (PHS) in automotive rear rail applications
[R].
Development trend and challenge of advanced high strength automobile steels
[J].
先进高强度汽车钢的发展趋势与挑战
[J].
Progress and perspective of advanced high strength automotive steel
[J].
先进高强度汽车用钢研究进展及展望
[J].
Effect of hot-dip galvanizing process on selective oxidation and galvanizability of medium manganese steel for automotive application
[J].A medium manganese steel with 7.5 wt.% Mn for automobile application was galvanized in a continuous Hot Dip Galvanizing (HDG) simulator under different galvanizing conditions. It was shown that the effects of dew point, annealing temperature and annealing atmosphere on the surface oxidation of steel could be comprehensively evaluated by the consideration of oxygen partial pressure P(O2). Although Mn2SiO4 was a thermodynamic stable phase when P(O2) varied from 10−28 to 10−21 atm, it was difficult to form Mn–Si–O composite oxide because there was no enrichment of silicon on the steel surface. So, this oxide was generally formed in the Fe substrate and had little effect on the galvanizability. With the increase in P(O2) above 10−25 atm, MnO particles in the form of the thermodynamic stable phase became coarser and tended to aggregate, which hindered the formation of a continuous inhibition layer, resulting in the defects of bare spots on the galvanized surface of the steel. When the oxygen partial pressure greater than 10−22 atm, film-like MnO layer was formed on the surface of steel sample, which obviously deteriorated the galvanizability. The galvanizability of the steel can be improved by the regulation of oxygen partial pressure; based on this, the reasonable zinc plating process parameters can be developed.
Effect of dew point on galvanizability in 4 mass% Al added low density steel
[J].
Effect of Mn and Cr on the selective oxidation, surface segregation and hot-dip Zn coatability
[J].
Investigation of selective oxidation and reaction wetting of Q&P steel under different dew point during continuous galvanizing
[J].
Interface microstructure and adhesion of zinc coatings on TRIP steels
[J].
Investigation on the coating adhesion of galvanized AHSS treated by oxidation-reduction process
[J].
Effect of selective oxidation behavior of high strength dual-phase steel surface on phosphating properties
[J].
高强双相钢表面选择性氧化行为对磷化性能的影响
[J].
Application of thermodynamics and Wagner model on two problems in continuous hot-dip galvanizing
[J].
Selective oxidation of ternary Fe-Mn-Si alloys during annealing process
[J].
Influence of Cr addition on selective oxidation behavior of Mn-added high-strength steel sheet
[J].
Effects of surface microstructure on selective oxidation morphology and kinetics in N2 + 5% H2 atmosphere with variable dew point temperature
[J].Starting surface microstructure has been shown to influence selective oxidation morphology and kinetics on cold-rolled grades of CMnSi advanced high strength steels (AHSSs). The research presented below examined this phenomenon after 120 and 1 800 s and under N-2 + 5%H-2 with 0 degrees C and -30 degrees C dewpoint temperatures (DPTs). Starting microstructures were altered by pre-annealing, which led to decarburization and grain growth. All samples were oxidized in a high temperature confocal scanning laser microscope (CSLM) at 850 degrees C. External oxides were subsequently examined using secondary electron scanning electron microscopy (SE SEM) and energy-dispersive spectroscopy (EDS). Cross-sections were produced and examined in a focused ion beam scanning electron microscope (FIB-SEM). Oxidizing atmosphere, surface microstructure, and time all influenced selective oxidation behavior. High and low DPT atmospheres had expected effects. Variation between microstructures was only apparent in 1 800 s samples oxidized in a high DPT atmosphere. Decarburized samples oxidized at higher DPTs exhibited surfaces covered with discrete iron nodules which may facilitate reactive zinc wetting.
Selective oxidation of a C-2Mn-1.3Si (wt pct) advanced high-strength steel during continuous galvanizing heat treatments
[J].
Selective oxidation of a 0.1C-6Mn-2Si third generation advanced high-strength steel during dew-point controlled annealing
[J].
Phase equilibrium calculation of water pressure control in hydrogen reduction system
[J].
氢还原体系控制水压的物相平衡计算
[J].
Effects of heating atmosphere on selective oxidation of a C-Mn-Si-Al high strength steel
[J].
加热气氛对一种C-Mn-Si-Al高强钢选择性氧化的影响
[J].
Effect of water pressure and soaking time on the selective oxidation of DP980 advanced high strength steel
[J].
Investigation of the effect of alloying elements and water vapor contents on the oxidation and decarburization of transformation-induced plasticity steels
[J].
Effect of internal oxidation on the weldability of CMnSi steels
[J].Surface oxide formation from the high alloying contents in advanced high strength steels is detrimental to their Zn coating quality. Selective oxidation by annealing is used in steel production to improve the wettability of Zn to steel surfaces in the continuous hot-dip galvanizing process. Internal (sub-surface) oxides formed in the annealing process suppress surface oxide formation. However, the effect of internal oxides on resistance spot welding has not been thoroughly investigated. In this study, internal oxides were found to cause the weld lobe to shift towards higher welding current by promoting shallow surface melting early in the weld cycle. Molten metal at the contact surface reduces contact resistance and heat input, delaying nugget growth. Dynamic resistance profile and heat input analysis results further confirm the surface melting mechanism in annealed samples.
The role of internal oxides on the liquid metal embrittlement cracking during resistance spot welding of the dual phase steel
[J].
The influence of modified annealing during the galvanizing process on the resistance spot welding of the CMn1.8Si advanced high strength steel
[J].
A study on selective oxidation behaviors of DP780 dual phase steels during hot-dip galvanizing process
[D].
热镀锌DP780双相钢的选择性氧化行为研究
[D].
Numerical simulation of internal oxidation of steels during annealing treatments
[J].
An empirical model for carbon diffusion in austenite incorporating alloying element effects
[J].
Effect of H2O(g) on decarburization of 55SiCr spring steel during the heating process
[J].
加热过程中H2O(g)对55SiCr弹簧钢脱碳的影响
[J].
Ellingham Diagram
[A].