铁基非晶合金具有优异的软磁性能和力学性能,包括低矫顽力(Hc)、低铁损、高导磁率(μ)及高强度[8~10],可用作变压器、互感器和永磁电机等铁芯材料,在结构材料领域也具有潜在的应用价值。添加少量前过渡金属元素(ETM,如:Zr、Nb、Mo、Hf、Ta、W等)和稀土元素(RE,如:Y、Nd、Dy、Er等)可有效提升铁基非晶合金的玻璃形成能力(GFA)、热稳定性及软磁性能和力学性能[11]。微合金化元素对非晶合金GFA的有利影响可归结为[4,5]:(1) 微量合金化元素的添加可增加非晶合金中原子的堆积密度,减少自由体积;(2) 合金化元素可增加非晶合金的短程有序结构,降低合金体系能量;(3) 微量合金化元素的添加可增强过冷液体的黏度,延缓合金过冷液体的结晶动力学过程。然而,由于微合金化元素的种类和数量对非晶合金GFA和性能的影响不尽相同,目前仍缺乏对非晶合金微合金化作用的深入研究和理解[4]。
本工作应用“团簇+连接原子”模型,通过分析非晶合金结构中团簇内部和团簇间原子的相互关联作用来建立非晶合金的结构-性能相关性,理解非晶合金的微合金化机理。并以Si、ETM和RE元素微合金化Fe-B基非晶合金进行实验验证。
1 “团簇+连接原子”模型
图1
图1
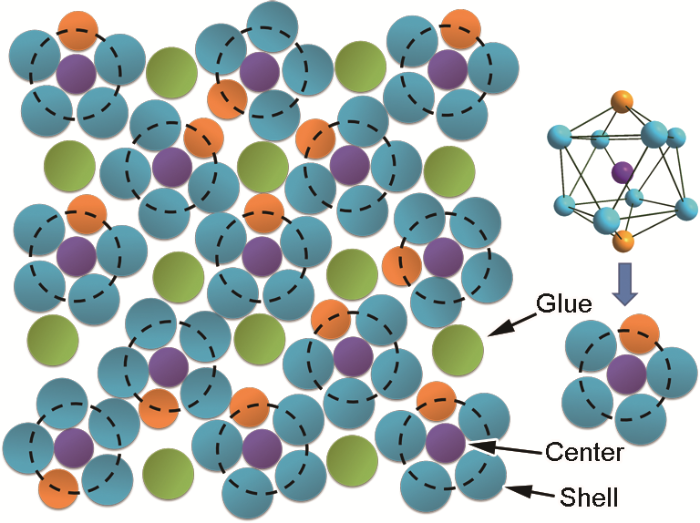
非晶合金的“团簇+连接原子”局域结构模型
Fig.1
2-dimensional schematic of the 3-dimensional amorphous structure with a set of constituent atoms (The face center cubic-like arrays of atomic clusters are circled by black dotted lines, leaving behind the glue atoms in the octahedral sites)
Color online
2 原子间关联作用
图2
图2
非晶合金中团簇内部和团簇间原子关联作用及合金化原子在模型结构中的位置示意图
Fig.2
Schematics of the intra- and inter-cluster correlations in the amorphous structures and the position of alloying atoms entering the model structure (C stand for the center atoms, S stand for the shell atoms, G stand for the glue atoms, A stand for the alloying atoms, subscripts 1 and 2 stand for their corresponding clusters)
Color online
(a) no minor alloying atom (b) minor alloying atom enters into the center position
(c) minor alloying atom enters into the shell position (d) minor alloying atom enters into the glue position
2.1 原子关联作用与非晶合金玻璃转变温度(Tg)的关系
在模型中,可通过分析非晶合金过冷液体中团簇间关联作用来理解非晶合金的玻璃化转变现象。根据Stillinger[26]关于脆性液体的统计力学理论,合金过冷液体剪切黏度(η)可以表示为:
考虑到化学浓度的贡献,式中,η0和B为正常数;ξ为与团簇间原子关联作用相关的域尺寸;ci和μi为第i个组分的浓度和化学势;N是原子个数;kB是Boltzmann常数;T为热力学温度。考虑到合金成分中元素间的混合焓,在合金的过冷液体中,如果团簇间存在强吸引关联作用,则液体中容易发生许多团簇和许多胶原子的协同运动。随着过冷度的增加,域的动态协同性将增强,并且在Tg处发生长距离团簇域协同运动的逾渗[27,28],与团簇间强吸引关联作用相关的大的域尺寸使得过冷液体具有较大的η(T),因此具有足够长的弛豫时间来动态捕获Tg[26]。因此,即使在常规的加热速率下,通过差示扫描量热仪(DSC)测量也可观察到非晶合金的玻璃转变现象。在给定的温度下,团簇间关联作用越强,域的尺寸越大,数密度也越大,非晶合金的Tg越高。如果团簇间的吸引关联作用较弱,较弱的域使得过冷液体的结构重排变得容易,原子的弛豫时间变短,过冷液体晶化变得容易,因此通过常规的加热速率,在非晶合金的DSC曲线中很难观察到Tg[29]。
2.2 原子关联作用与非晶合金晶化温度(Tx)的关系
由于非晶合金与其结晶后晶态合金的中长程结构和局部成分差异较大,因此,在非晶合金晶化过程中,团簇内部原子间强的关联作用被破坏,部分原子从结晶相中排出,这与非晶合金的Tx相关。
2.3 原子关联作用与非晶合金GFA的关系
一般来说,仅用过冷液体的稳定性变化尚不能完全解释非晶合金的GFA,实际上,非晶合金GFA的差异通常与约化玻璃转变温度(Trg)一致。因此,结晶相的动力学过程对大块金属玻璃的形成起着决定性的作用。根据经典成核理论,单位体积的晶体成核率(I)可表示为[30]:
式中,A为动力学常数;ΔG*为能量势垒。对液体形核的贡献包括热力学参量ΔG*和动力学参量η(T)。ΔG*可表示为:
式中,ΔGl,s为液相和晶体相的Gibbs自由能差,σ为晶体和液体的界面张力。在相同的合金体系中,成分的小范围变化(微合金化)对过冷液体的晶化产物影响较小,因此,在过冷液体中,相同体系的合金的ΔGl,s差别较小。σ可表示为:
因此,团簇间原子关联作用,即:η(T)在微合金化元素对非晶合金GFA影响方面起着至关重要的作用。在具有强团簇间原子关联作用的过冷液体中,较大的η(T)可以限制晶体的成核和生长过程,从而有助于提升合金的GFA。但是,如果团簇内部或团簇间原子间关联作用过强,过冷液体中很容易发生局部原子有序化,导致液相线温度急剧升高。因此,随着微合金化元素含量的增加,非晶合金的GFA先增加后降低。合金化元素的最佳含量可通过计算单位团簇式所具有的价电子数(e/u)进行估算[22]。
2.4 原子关联作用与非晶合金力学性能的关系
流动阻力是了解非晶合金变形行为的关键[33]。已知非晶合金的屈服伴随着剪切带内温度的显著升高,这导致剪切带中的黏度显著降低。在施加应力的情况下,剪切带内的流变启动将主要通过关联域内弹性变形及其边界结构的剪切运动来响应。因此,流变应力应与团簇间原子关联作用相关。对于共价材料,从微观上硬度可以定义为[34]:“单位面积上的化学键对金刚石压头的阻抗能力”。金属-类金属型非晶合金具有类共价材料的特征,由于团簇内部原子间为强关联作用,而团簇与团簇间原子的关联作用较弱,因此在对非晶合金进行压痕测试时,原子键的优先断裂位置应该在近邻团簇与团簇的壳层原子之间,即团簇间原子关联的位置,这也可解释非晶合金断裂强度与Tg之间的近似线性关系[35]。
总体来说,基于非晶合金的局域结构模型,Tg、GFA、显微硬度和强度主要由团簇间原子关联作用决定,团簇内部原子关联作用与非晶合金的热稳定性相关,如:Tx。
3 非晶合金的微合金化作用
根据“团簇+连接原子”模型,微合金化元素在模型结构中的位置主要有3种,即:团簇中心、团簇壳层和连接原子,见图2b~d。
3.1 团簇中心
由于非晶合金中团簇中心原子与壳层原子之间的吸引关联作用较强,因此,只有当微合金化原子与壳层原子之间的吸引关联作用(如:焓)大于原团簇心部与壳层原子之间时关联作用时,微合金化原子才有可能取代原团簇的中心位置(图2b)或与原壳层原子构建新的团簇,在这种情况下,金属玻璃的热稳定性(如:Tx)显著提高。
3.2 团簇壳层
当团簇中心原子与微合金化原子之间有强的吸引关联作用时,微合金化原子倾向于进入到团簇的壳层位置(图2c)。在这一情况下,非晶合金的热稳定性会得到提高。但只有当微合金化原子与近邻团簇的壳层原子有较强吸引关联作用时,非晶合金才会出现明显的玻璃化转变现象,具有较高的GFA、显微硬度和强度。
3.3 连接原子
这里主要包含2种情况:(1) 当微合金化原子与团簇中心原子的吸引关联作用较弱时,微合金化原子倾向于进入到连接原子位置,此时合金化原子对非晶合金热稳定性的影响较弱。并且,只有当微合金化原子与团簇的壳层原子有较强的吸引关联作用时,非晶合金才会出现明显的玻璃化转变现象,具有较高的GFA、显微硬度和强度;(2) 当微合金化原子的半径较大,团簇中心原子与微合金原子的吸引关联作用不足以将微合金化原子吸引至壳层位置时,合金化原子倾向于进入到连接原子位置(图2d)。此时,即使微合金化原子与壳层原子间的吸引关联作用较弱,连接原子位置的大原子也会阻碍合金过冷液体的流动,增加过冷液体的黏度,非晶合金同样会出现明显的玻璃化转变现象和较高的GFA。
4 Fe-B基非晶合金局域结构模型的建立
Fe-B二元及Fe-B基多元非晶合金具有明显的近程有序特征[36]。在非晶态结构中,B原子周围的原子团簇与Fe2B相的原子团簇结构相似[15,37]。在四方Fe2B相中,B原子只占据一个原子位置,以B原子为中心,近邻原子为壳层,可构建附2个半八面体Archimedes反三棱柱团簇[B-B2Fe8],其中B和B2Fe8分别代表团簇的中心原子和壳层原子,下标代表原子个数。基于结构特征,[B-B2Fe8]原子簇被认为是最有可能出现在Fe-B非晶合金中所有可能结构中的基础结构。对于理想非晶合金,其单位团簇式中e/u接近常数24[38]。因此,选择1个Fe原子作为连接原子,可设计具有较高GFA的Fe-B二元非晶合金团簇式成分:[B-B2Fe8]Fe(Fe75B25)。
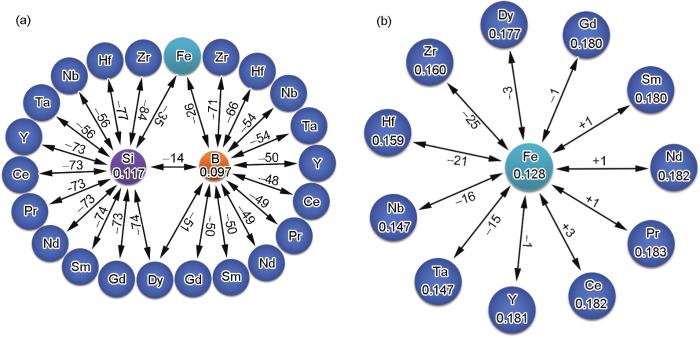
在非晶合金的过冷液体中,化学短程有序化的趋势与组成元素之间的混合焓(ΔH)有关[39]。在Fe-B-Si系统中,ΔHFe-B=-26 kJ/mol,ΔHFe-Si=-35 kJ/mol,ΔHB-Si=-14 kJ/mol[40],见图3。较强的负混合焓意味着在过冷液体和非晶结构中,B原子和Si原子都会表现出对特定最近邻Fe环境的偏好。如果Si原子占据中心B位形成团簇,[B-B2Fe8]原子团簇的结构能将进一步降低。因此,在Fe-B-Si三元合金中,[Si-B2Fe8]Fe(Fe75B16.7Si8.3)合金成分具有最佳GFA,这一结果与之前报道的Fe75B16Si9最佳GFA成分接近[41]。
图3
图3
不同元素之间的混合焓及各自的原子半径
Fig.3
The equal-atomic ratio enthalpy of mixing (ΔH, kJ/mol) data of B, Si (a) and Fe (b) with the early transition metals (ETMs, Zr, Hf, Nb and Ta) and rare earth elements (RE, Y, Ce, Pr, Nd, Sm, Gd and Dy) (The Goldschmidt radii (nm) of these elements are also presented)
Color online
考虑到ETM (Zr、Hf、Nb、Ta)和RE (Y、Ce、Pr、Nd、Sm、Gd、Dy)与B、Si的混合焓较负,选择它们作为微合金化合金元素。图3显示了B、Si和Fe与微合金化元素间的混合焓[40]:ΔHB(or Si)-ETM和ΔHB(or Si)-RE为-84~-48 kJ/mol;ΔHFe-ETM为-25~-15 kJ/mol;ΔHFe-RE为-3~+3 kJ/mol。图3中还包括了各个原子的Goldschmidt半径[42]。合金化原子的半径在0.147~0.181 nm之间变化。由于ETM/RE原子与Si原子之间的ΔH较大,ETM和RE原子更倾向于与Si原子近邻,占据壳层位置。因此,四元非晶合金的结构和成分可表示为:[Si-B2(Fe, ETM/RE)8]Fe。
5 实验方法
以纯度99.99% (质量分数,下同)的Fe和Si、99.5%的B、99.95%的Zr、Hf、Nb和Ta及99.0%的Y、Ce、Pr、Nd、Sm、Gd和Dy单质为原料配置样品。在纯Ar气氛保护下反复熔炼母合金锭4次,以保证其成分均匀性;采用单辊甩带技术制备1.0 mm宽、0.02 mm厚的条带样品,铜辊表面线速率为40 m/s;由铜模吸铸法制备直径1.0~3.0 mm的棒状样品;用D8 Focus 型X射线衍射仪(XRD,CuKα,波长λ=0.15406 nm)测试条带和棒状样品的晶体结构,棒状样品均机械破碎成粉末后进行测量;条带样品的热分析在Q100型差示扫描量热仪(DSC)和Q600差热分析仪(DTA)上进行,升温速率均为0.33 K/s;利用HRS-150型Vickers硬度测试仪对直径为1.0 mm的棒状非晶合金进行了Vickers硬度测量,载荷为1 kg,保压时间为15 s。
6 实验验证
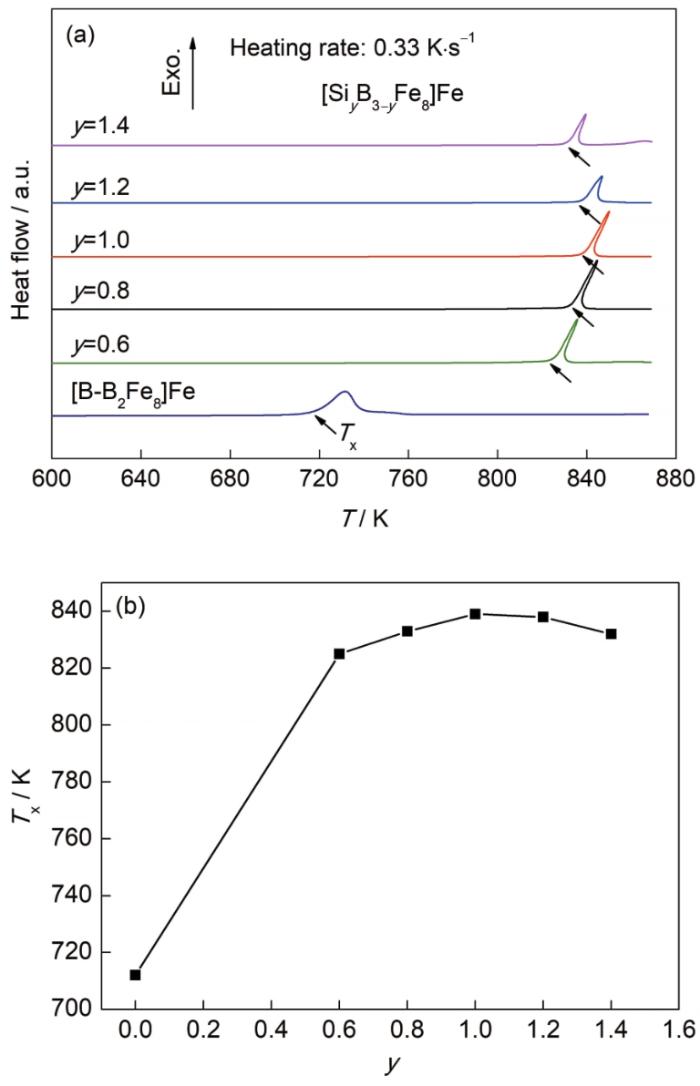
图4a给出了随Fe75B25和系列Si成分(y)变化的Fe-B-Si三元非晶合金的DSC曲线。当团簇式中Si原子个数≤1时,Si原子替代团簇中心位置的B原子,形成[(B, Si)-B2Fe8]Fe团簇式;当团簇式中Si原子个数>1时,1个Si原子替代中心位置的B原子,剩余的Si原子替代壳层位置的B原子,形成[Si-(B, Si)2Fe8]Fe团簇式。可知,当Si替代B后,非晶合金的Tx提升明显,见图4b。y=1时(团簇中心B原子恰好被一个Si原子替代)非晶合金的Tx具有最大值839 K。这主要是由于团簇心部Si与壳层Fe和B都具有较强的负混合焓,团簇心部B原子被Si替代后可大幅增强团簇内部原子的吸引关联作用,从而提升合金的Tx。但当Si原子继续增加,壳层位置的B原子被Si替代后,由于心部Si原子与壳层Si原子的吸引关联作用较弱,导致团簇内部原子吸引关联作用重新降低,Tx开始下降。由于Si的替代对团簇间原子吸引关联作用的影响较小(主要受到团簇心部原子与相邻团簇壳层(次近邻) Fe原子关联作用改变的影响),因此,对合金GFA的提升有限。由于Fe-B二元和Fe-B-Si三元非晶合金结构中团簇间原子吸引关联作用较弱,因此,很难获得块体非晶合金,并且在非晶合金的DSC曲线中也很难观察到明显玻璃转变现象。
图4
图4
Fe-B和Fe-B-Si非晶合金的DSC曲线及非晶合金的晶化温度(Tx)随[SiyB3-yFe]Fe团簇式中Si含量(y)的变化
Fig.4
DSC curves of Fe-B and Fe-B-Si ternary amorphous ribbons (T—temperature) (a) and crystallization temperature (Tx) as a function of Si content (y) in [SiyB3-yFe]Fe cluster formula (b)
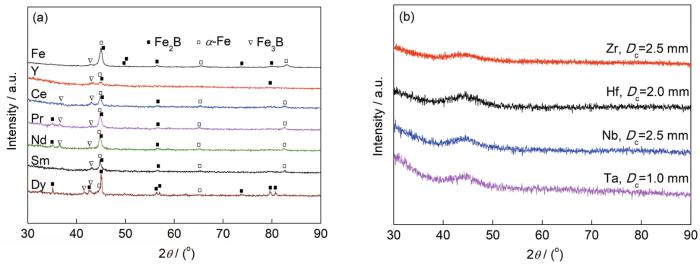
图5给出了直径(D)为1.0 mm的[Si-B2Fe8]Fe和[Si-B2Fe7.6RE0.4]Fe样品及非晶形成临界直径(Dc)样品[Si-B2Fe7.6ETM0.4]Fe的XRD谱。可见,Fe-B-Si及RE微合金化样品均不能形成块体非晶合金(图5a),但可形成条带非晶样品。ETM微合金化样品可形成Dc为1.0~2.5 mm的棒状块体非晶合金[29,43~45],见图5b。表明RE微合金化元素的加入并没有提升样品的GFA,而ETM微合金化元素的加入大幅提升了样品的GFA。图6给出了非晶条带样品的DSC曲线,通过分析DSC曲线可获得非晶合金的Tg和Tx,总结结果见表1。从表1可知,在RE微合金化的非晶合金中没有观察到Tg,但非晶合金的Tx得到大幅提升。
图5
图5
直径(D)为1 mm的[Si-B2Fe8]Fe和[Si-B2Fe7.6RE0.4]Fe样品及非晶形成临界直径(Dc)样品[Si-B2Fe7.6ETM0.4]Fe的XRD谱
Fig.5
XRD spectra of the [Si-B2Fe8]Fe and [Si-B2Fe7.6RE0.4]Fe rod samples with diameter (D) of 1.0 mm (a), and the [Si-B2Fe7.6ETM0.4]Fe alloys with critical diameter for glass formation (Dc) (b)
图6
图6
[Si-B2Fe8]Fe、[Si-B2Fe7.6RE0.4]Fe和[Si-B2Fe7.6ETM0.4]Fe非晶条带样品的DSC曲线
Fig.6
DSC curves of [Si-B2Fe8]Fe, [Si-B2Fe7.6RE0.4]Fe (a) and [Si-B2Fe7.6ETM0.4]Fe (b) amorphous ribbons (Tg—glass transition temperature)
表1 非晶合金的团簇式、化学成分、Dc、玻璃转变温度(Tg)、Tx和Vickers硬度
Table 1
| Cluster formula | Composition | Dc | Tg | Tx | Vickers hardness |
|---|---|---|---|---|---|
| (atomic fraction / %) | mm | K | K | HV | |
| [B-B2Fe8]Fe | Fe75B25 | <1.0 | - | 712 | - |
| [(B0.4Si0.6)-B2Fe8]Fe (y=0.6) | Fe75B20Si5 | <1.0 | - | 825 | - |
| [(B0.2Si0.8)-B2Fe8]Fe (y=0.8) | Fe75B18.33Si6.67 | <1.0 | - | 833 | - |
| [Si-B2Fe8]Fe (y=1.0) | Fe75B16.67Si8.33 | <1.0 | - | 839 | - |
| [Si-B1.8Si0.2Fe8]Fe (y=1.2) | Fe75B15Si10 | <1.0 | - | 838 | - |
| [Si-B1.6Si0.4Fe8]Fe (y=1.4) | Fe75B13.33Si11.67 | <1.0 | - | 832 | - |
| [Si-B2Fe7.6Y0.4]Fe (RE=Y) | Fe71.67B16.67Si8.33Y3.33 | <1.0 | - | 923 | - |
| [Si-B2Fe7.6Dy0.4]Fe (RE=Dy) | Fe71.67B16.67Si8.33Dy3.33 | <1.0 | - | 924 | - |
| [Si-B2Fe7.6Ce0.4]Fe (RE=Ce) | Fe71.67B16.67Si8.33Ce3.33 | <1.0 | - | 902 | - |
| [Si-B2Fe7.6Nd0.4]Fe (RE=Nd) | Fe71.67B16.67Si8.33Nd3.33 | <1.0 | - | 917 | - |
| [Si-B2Fe7.6Pr0.4]Fe (RE=Pr) | Fe71.67B16.67Si8.33Pr3.33 | <1.0 | - | 914 | - |
| [Si-B2Fe7.6Sm0.4]Fe (RE=Sm) | Fe71.67B16.67Si8.33Sm3.33 | <1.0 | - | 898 | - |
| [Si-B2Fe7.6Gd0.4]Fe (RE=Gd) | Fe71.67B16.67Si8.33Gd3.33 | <1.0 | - | 921 | - |
| Cluster formula | Composition | Dc | Tg | Tx | Vickers hardness |
| (atomic fraction / %) | mm | K | K | HV | |
| [Si-B2Fe7.8Zr0.2]Fe (ETM=Zr, z=0.2) | Fe73.33B16.67Si8.33Zr1.67 | 2.0 | 839 | 873 | 1120±9 |
| [Si-B2Fe7.7Zr0.3]Fe (ETM=Zr, z=0.3) | Fe72.50B16.67Si8.33Zr2.50 | 2.5 | 846 | 882 | 1131±7 |
| [Si-B2Fe7.6Zr0.4]Fe (ETM=Zr, z=0.4) | Fe71.67B16.67Si8.33Zr3.33 | 2.5 | 851 | 888 | 1149±7 |
| [Si-B2Fe7.5Zr0.5]Fe (ETM=Zr, z=0.5) | Fe70.83B16.67Si8.33Zr4.17 | 2.0 | 854 | 891 | 1145±8 |
| [Si-B2Fe7.4Zr0.6]Fe (ETM=Zr, z=0.6) | Fe70B16.67Si8.33Zr5 | 1.5 | 868 | 903 | 1156±7 |
| [Si-B2Fe7.3Zr0.7]Fe (ETM=Zr, z=0.7) | Fe69.17B16.67Si8.33Zr5.83 | 1.5 | 875 | 909 | 1170±10 |
| [Si-B2Fe7.2Zr0.8]Fe (ETM=Zr, z=0.8) | Fe68.33B16.67Si8.33Zr6.67 | 1.0 | 878 | 918 | 1184±8 |
| [Si-B2Fe7.1Zr0.9]Fe (ETM=Zr, z=0.9) | Fe67.5B16.67Si8.33Zr7.5 | 1.0 | 890 | 922 | 1195±13 |
| [Si-B2Fe7.0Zr1.0]Fe (ETM=Zr, z=1.0) | Fe66.67B16.67Si8.33Zr8.33 | <1.0 | 895 | 925 | 1197±15 |
| [Si-B2Fe7.8Hf0.2]Fe (ETM=Hf, z=0.2) | Fe73.33B16.67Si8.33Hf1.67 | 1.0 | 840 | 872 | 1115±13 |
| [Si-B2Fe7.7Hf0.3]Fe (ETM=Hf, z=0.3) | Fe72.50B16.67Si8.33Hf2.50 | 2.5 | 852 | 885 | 1121±19 |
| [Si-B2Fe7.6Hf0.4]Fe (ETM=Hf, z=0.4) | Fe71.67B16.67Si8.33Hf3.33 | 2.0 | 854 | 887 | 1153±8 |
| [Si-B2Fe7.5Hf0.5]Fe (ETM=Hf, z=0.5) | Fe70.83B16.67Si8.33Hf4.17 | 1.5 | 858 | 893 | 1138±9 |
| [Si-B2Fe7.4Hf0.6]Fe (ETM=Hf, z=0.6) | Fe70B16.67Si8.33Hf5 | 1.0 | 861 | 901 | 1168±8 |
| [Si-B2Fe7.3Hf0.7]Fe (ETM=Hf, z=0.7) | Fe69.17B16.67Si8.33Hf5.83 | 1.0 | 870 | 908 | 1174±9 |
| [Si-B2Fe7.2Hf0.8]Fe (ETM=Hf, z=0.8) | Fe68.33B16.67Si8.33Hf6.67 | <1.0 | 876 | 911 | - |
| [Si-B2Fe7.1Hf0.9]Fe (ETM=Hf, z=0.9) | Fe67.5B16.67Si8.33Hf7.5 | <1.0 | 886 | 918 | - |
| [Si-B2Fe7.8Nb0.2]Fe (ETM=Nb, z=0.2) | Fe73.33B16.67Si8.33Nb1.67 | 1.0 | - | 861 | 1087±12 |
| [Si-B2Fe7.7Nb0.3]Fe (ETM=Nb, z=0.3) | Fe72.5B16.67Si8.33Nb2.5 | 2.0 | 835 | 869 | 1090±1 |
| [Si-B2Fe7.6Nb0.4]Fe (ETM=Nb, z=0.4) | Fe71.67B16.67Si8.33Nb3.33 | 2.5 | 843 | 873 | 1108±11 |
| [Si-B2Fe7.5Nb0.5]Fe (ETM=Nb, z=0.5) | Fe70.83B16.67Si8.33Nb4.17 | 2.5 | 845 | 881 | 1113±4 |
| [Si-B2Fe7.4Nb0.6]Fe (ETM=Nb, z=0.6) | Fe70B16.67Si8.33Nb5 | 2.0 | 853 | 885 | 1126±9 |
| [Si-B2Fe7.3Nb0.7]Fe (ETM=Nb, z=0.7) | Fe69.17B16.67Si8.33Nb5.83 | 1.5 | 854 | 895 | 1150±13 |
| [Si-B2Fe7.2Nb0.8]Fe (ETM=Nb, z=0.8) | Fe68.33B16.67Si8.33Nb6.67 | 1.5 | 856 | 902 | 1172±20 |
| [Si-B2Fe7.1Nb0.9]Fe (ETM=Nb, z=0.9) | Fe67.5B16.67Si8.33Nb7.5 | 1.5 | 862 | 911 | 1173±25 |
| [Si-B2Fe7.0Nb1.0]Fe (ETM=Nb, z=1.0) | Fe66.67B16.67Si8.33Nb8.33 | 1.5 | 875 | 917 | 1188±28 |
| [Si-B2Fe6.8Nb1.2]Fe (ETM=Nb, z=1.2) | Fe65B16.67Si8.33Nb10 | 1.0 | 889 | 930 | - |
| [Si-B2Fe7.8Ta0.2]Fe (ETM=Ta, z=0.2) | Fe73.33B16.67Si8.33Ta1.67 | <1.0 | - | 858 | - |
| [Si-B2Fe7.7Ta0.3]Fe (ETM=Ta, z=0.3) | Fe72.5B16.67Si8.33Ta2.5 | <1.0 | - | 865 | - |
| [Si-B2Fe7.6Ta0.4]Fe (ETM=Ta, z=0.4) | Fe71.67B16.67Si8.33Ta3.33 | 1.0 | 843 | 873 | 1117±6 |
| [Si-B2Fe7.5Ta0.5]Fe (ETM=Ta, z=0.5) | Fe70.83B16.67Si8.33Ta4.17 | 1.0 | 850 | 884 | 1130±10 |
| [Si-B2Fe7.4Ta0.6]Fe (ETM=Ta, z=0.6) | Fe70B16.67Si8.33Ta5 | 1.0 | 856 | 889 | 1143±10 |
| [Si-B2Fe7.3Ta0.7]Fe (ETM=Ta, z=0.7) | Fe69.16B16.67Si8.33Ta5.83 | 1.0 | 856 | 891 | 1154±11 |
| [Si-B2Fe7.2Ta0.8]Fe (ETM=Ta, z=0.8) | Fe68.33B16.67Si8.33Ta6.67 | <1.0 | 859 | 896 | - |
| [Si-B2Fe7.1Ta0.9]Fe (ETM=Ta, z=0.9) | Fe67.5B16.67Si8.33Ta7.5 | <1.0 | 863 | 910 | - |
| [Si-B2Fe7.0Ta1.0]Fe (ETM=Ta, z=1.0) | Fe66.67B16.67Si8.33Ta8.33 | <1.0 | 874 | 913 | - |
在ETM微合金化的非晶合金中可观察到明显的Tg,Tx也得到提升。
由于团簇中心Si与壳层位置的RE和ETM都具有较强的负混合焓,因此,这些微合金化元素的加入均可提升团簇内部原子的吸引关联作用,从而提升非晶合金的Tx,RE元素对非晶合金的Tx提升幅度更大,这主要归因于其大的原子半径所导致的原子扩散阻力增加。由于ETM与Fe之间有较强的负混合焓,因此ETM的加入,可提升ETM原子与相邻团簇壳层Fe原子之间的吸引关联作用,从而增加团簇间原子的关联作用,增加合金的GFA,并且从非晶合金的DSC曲线中也可观察到明显的Tg。但RE与Fe之间的混合焓接近0,因此,微合金化元素RE的加入对团簇间原子的关联作用影响较小,并不能有效提升非晶合金的GFA,见图7。
图7
图7
Fe-B-Si、Fe-B-Si-ETM和Fe-B-Si-RE非晶合金团簇内部和团簇间原子的关联作用简化图
Fig.7
Schematics of the anisotropic inter-atomic and inter-cluster correlation of Fe-B-Si (a), Fe-B-Si-ETM (b) and Fe-B-Si-RE (c) amorphous alloys
Color online
图8
图8
[Si-B2Fe8-zETMz]Fe非晶合金的Tg、Tx和Vickers硬度随合金元素含量变化关系曲线
Fig.8
The changes of Tg and Tx (a) and Vickers hardness (b) with the ETMs (Zr, Hf, Nb and Ta) content (z) in [Si-B2Fe8-zETMz]Fe cluster formula
其它非晶合金的微合金化作用也可通过模型中原子间的关联作用进行解释。添加RE元素可大幅提升Fe-B二元非晶合金的GFA[46],这可能主要归因于B-RE的原子间吸引关联作用弱于Si-RE,而RE的原子半径又过大,所以RE原子倾向于进入到连接原子位置。连接原子位置的RE大原子会阻碍过冷液体中团簇的流动,从而增加了液体的黏度,提升了合金的GFA。可以预见,在Fe-B合金中同时加入RE和ETM是提升其GFA的有效手段[47]。但由于这些非晶合金中RE的作用机制并非来自原子间的化学关联作用,而是纯粹源自原子尺寸的影响,因此在其过冷液体中表现出不同的热力学行为,这些非晶合金在DSC测量过程中表现出的“双Tg”现象可能与2种作用机制的先后启动有关。对于Fe-P和Fe-C系非晶合金,由于其源自Fe3P和Fe3C的局域团簇结构分别为[P-Fe9]和[C-Fe9],连接原子也主要由P或C构成。由于P-Fe和C-Fe之间有较强的负混合焓,团簇间原子吸引关联作用较强,因此,相比于Fe-B系非晶合金,Fe-P和Fe-C系非晶合金的玻璃转变现象更加明显,GFA也更高[48~50]。对于Cu-Zr二元非晶合金,Al、Be、Si和Sn微合金化元素的加入可有效增加非晶合金的GFA和显微硬度[51~53],这主要是因为这些合金化元素主要进入到连接原子的位置,与[Cu-Cu7Zr5]团簇壳层位置的Zr有较强的负混合焓,从而增加了团簇间的吸引关联作用。Al替代Ni-Zr二元非晶合金的Zr后,可使非晶合金的Tg和Tx线性增加[54],这主要是由于Al-Zr和Al-Ni之间的负混合焓强于Ni-Zr,Al替代[Ni-Ni2Zr8]团簇壳层位置的Zr后,团簇内部和团簇间原子吸引关联作用同时增强所致。
7 结论
通过建立非晶合金的局域结构模型,分析模型结构中原子间关联作用的变化,可对非晶合金的微合金化作用进行解释。根据微合金化元素的性质,其进入模型结构中的位置可分为团簇中心、团簇壳层和连接原子3类。非晶合金的GFA和Tg及其成分相关的显微硬度和强度归因于团簇间原子的关联作用。非晶合金的热稳定性与团簇内部原子关联作用相关。实验验证结果表明,Si、ETM和RE及合金化元素的加入均使非晶合金局域结构中团簇内部原子吸引关联作用增强,非晶合金的热稳定性得到提高。但由于含RE元素非晶合金结构中团簇间的原子吸引关联作用较弱,因此无法在Fe-B-Si-RE合金中获得块体非晶合金及观察到明显的玻璃转变现象,实验结果与理论相吻合。该理论还可用于解释其它非晶合金的微合金化作用。
参考文献
Effect of boron on grain-boundaries in Ni3Al
[J].
Grain refinement of alloys by inoculation of melts
[J].
Roles of minor additions in formation and properties of bulk metallic glasses
[J].
Role of minor alloying additions in formation of bulk metallic glasses: A review
[J].
Unusual glass-forming ability of bulk amorphous alloys based on ordinary metal copper
[J].
Fe-based bulk metallic glasses with diameter thickness larger than one centimeter
[J].
Magnetic amorphous alloys: Physics and technological applications
[J].
Amorphous magnetic materials—A history
[J].
Amorphous and nanocrystalline materials for applications as soft magnets
[J].
Iron-based bulk metallic glasses
[J].
Structure of Metallic Glasses
[M].
Structure of binary metallic glasses
[J].
Iron-boron metallic glasses
[J].
Short-range order in a partially crystallized Fe0.86B0.14 amorphous alloy: A comparison between spin-echo NMR and Mössbauer-effect studies
[J].
Mössbauer study of glassy alloys (Fe-Mo)80B20
[J].
The structure of glassy metallic alloys
[J].
Ductility improvement of amorphous steels: Roles of shear modulus and electronic structure
[J].
Ternary bulk metallic glasses formed by minor alloying of Cu8Zr5 icosahedron
[J].
Ni-Ta binary bulk metallic glasses
[J].
Understanding the Ni-Nb-Zr BMG composition from a binary eutectic Ni-Nb icosahedral cluster
[J].
From clusters to phase diagrams: Composition rules of quasicrystals and bulk metallic glasses
[J]. J
Composition design procedures of Ti-based bulk metallic glasses using the cluster-plus-glue-atom model
[J].
Atomic-level structure and structure-property relationship in metallic glasses
[J].
Atomic packing and short-to-medium-range order in metallic glasses
[J].Unlike the well-defined long-range order that characterizes crystalline metals, the atomic arrangements in amorphous alloys remain mysterious at present. Despite intense research activity on metallic glasses and relentless pursuit of their structural description, the details of how the atoms are packed in amorphous metals are generally far less understood than for the case of network-forming glasses. Here we use a combination of state-of-the-art experimental and computational techniques to resolve the atomic-level structure of amorphous alloys. By analysing a range of model binary systems that involve different chemistry and atomic size ratios, we elucidate the different types of short-range order as well as the nature of the medium-range order. Our findings provide a reality check for the atomic structural models proposed over the years, and have implications for understanding the nature, forming ability and properties of metallic glasses.
Relaxation and flow mechanisms in "fragile" glass-forming liquids
[J].
Spatially heterogeneous dynamics in supercooled liquids
[J].
Two-order-parameter model of the liquid-glass transition. II. Structural relaxation and dynamic heterogeneity
[J].
Formation and structure-property correlation of new bulk Fe-B-Si-Hf metallic glasses
[J].
Crystal nucleation in liquids and glasses
[J].
A structural model for the solid-liquid interface in monatomic systems
[J].
The nature of the glassy state and the behavior of liquids at low temperatures
[J].
Mechanical behavior of amorphous alloys
[J].
Hardness of covalent crystals
[J].Based on the idea that the hardness of covalent crystal is intrinsic and equivalent to the sum of the resistance to the indenter of each bond per unit area, a semiempirical method for the evaluation of hardness of multicomponent crystals is presented. Applied to beta-BC2N crystal, the predicted value of hardness is in good agreement with the experimental value. It is found that bond density or electronic density, bond length, and degree of covalent bonding are three determinative factors for the hardness of a polar covalent crystal. Our method offers the advantage of applicability to a broad class of materials and initializes a link between macroscopic property and electronic structure from first principles calculation.
The elastic properties, elastic models and elastic perspectives of metallic glasses
[J].
Models for the structure of amorphous metals
[A].
Topics in Applied Physics
[M].
Composition formulas of Fe-B binary amorphous alloys
[J].
The thermoelectric power of the metallic glass Ca0.8Al0.2
[J].
Effect of Fe-B-Si composition on maximum thickness for casting amorphous metals
[J].
Composition design and optimization of Fe-B-Si-Nb bulk amorphous alloys
[J].
Fe-B-Si-Nb块体非晶合金的成分设计与优化
[J].
Fe-B-Si-Zr soft magnetic bulk glassy alloys
[J].
Composition design of Fe-B-Si-Ta bulk amorphous alloys based on cluster + glue atom model
[J].
基于团簇+连接原子模型的Fe-B-Si-Ta块体非晶合金的成分设计
[J].
Soft magnetic properties and glass formability of Y-Fe-B-M bulk metals (M=Al, Hf, Nb, Ta, and Ti)
[J].
Electronic-structure origin of the glass-forming ability and magnetic properties in Fe-RE-B-Nb bulk metallic glasses
[J].
Effects of Mo addition on thermal stability and magnetic properties of a ferromagnetic Fe75P10C10B5 metallic glass
[J].
Structural amorphous steels
[J].Recent advancement in bulk metallic glasses, whose properties are usually superior to their crystalline counterparts, has stimulated great interest in fabricating bulk amorphous steels. While a great deal of effort has been devoted to this field, the fabrication of structural amorphous steels with large cross sections has remained an alchemist's dream because of the limited glass-forming ability (GFA) of these materials. Here we report the discovery of structural amorphous steels that can be cast into glasses with large cross-section sizes using conventional drop-casting methods. These new steels showed interesting physical, magnetic, and mechanical properties, along with high thermal stability. The underlying mechanisms for the superior GFA of these materials are discussed.
Fe-based bulk metallic glasses: Brittle or ductile?
[J].
Thermal and mechanical properties of Cu-Zr-Al bulk metallic glasses
[J]. J
Thermal and elastic properties of Cu-Zr-Be bulk metallic glass forming alloys
[J].
Effects of a minor addition of Si and/or Sn on formation and mechanical properties of Cu-Zr-Ti bulk metallic glass
[J].
Formation and mechanical properties of Zr-Ni-Al glassy alloys with high glass-forming ability
[J].AbstractThe addition of Al to a binary Zr75Ni25 alloy greatly increases the stabilization of the supercooled liquid as well as the glass-forming ability (GFA) in Zr75−xNi25Alx (x = 0–24) alloy series. The large supercooled liquid region (ΔTx) of over 80 K and critical sample diameter (dc) for glass formation above 12 mm were obtained in a wide composition range of x = 11–19. The largest dc of 15 mm was fabricated for an off-eutectic Zr60Ni25Al15 alloy, which has the largest ΔTx of 93 K and γ value of 0.396. The bulk glassy alloys exhibited excellent mechanical properties, i.e., high compressive fracture strength of 1662–1958 MPa and large compressive plastic strain of 3.0–4.7%.]]>