γ-TiAl合金是一种金属间化合物,其密度约为4.0 g/cm3 [1]。有序的晶体结构赋予了γ-TiAl合金独特的性能,如较高的使用温度、高比强度、高比模量,优异的抗蠕变性能和抗氧化性能,以及良好的结构稳定性,一直被国内外视为极具应用前景的航空航天材料[2,3]。根据成分的不同可以将γ-TiAl合金分为传统γ-TiAl (CG)和β凝固γ-TiAl (BSG) 2类[4]。CG合金使用温度一般不超过750 ℃,且凝固过程经过包晶反应区,导致铸态组织粗大[5],而BSG合金凝固过程无包晶转变,铸锭组织均匀细小[6]。BSG合金通常含有一种或多种β相稳定元素以及较低的Al含量(42%~45%,原子分数)。相较于CG合金,BSG合金通过添加β相稳定元素,提高了合金的使用温度和高温下的延展性,扩大了合金的热加工工艺窗口,提升了合金的热加工性能,使其能够在一定条件下进行热加工变形[7~9]。
Nb是广泛应用于γ-TiAl合金中的β相稳定元素,它能够提高合金高温强度、抗蠕变性能和抗氧化性能[10~12]。然而,在TNM (Ti-43.5Al-4Nb-1Mo-0.1B,原子分数,%,除另有说明外,文中所有成分均为原子分数)和TNB (Ti-45Al-5/8Nb-0.2B-0.2C)合金中,高的Nb含量使得室温组织保留了部分脆性βo相,在长期服役过程中容易析出ωo (Ti4Al3Nb)相。这不但降低了合金的延展性,而且破坏了片层结构的稳定性[13~16]。Mn的β相稳定效应约为Nb的3倍[17],可以促进位错滑移,增强机械孪晶,并提升合金的变形能力[18,19]。同时,少量的Mn元素加入可以抑制含Nb γ-TiAl合金中ωo相的形成[20]。因此,近期本课题组[21,22]设计了一种新型Ti-Al-Mn-Nb系的β凝固γ-TiAl合金,该类合金在750、800和850 ℃均具有良好的抗氧化性能,其高温抗氧化能力较Ti-42Al-5Mn-1W有了进一步提升。
对于新型的β凝固γ-TiAl合金体系,研究合金的相变行为和组织演变规律对于制定合理的热加工和热处理工艺具有极其重要的指导意义[23,24]。尽管Schuster和Palm[25]于2006年给出了权威的Ti-Al二元相图,然而金属元素的加入不仅会影响γ-TiAl合金的组织和性能,还会改变其凝固路径。因此,很难通过Ti-Al二元相图确定多组分γ-TiAl合金的相变路径和凝固组织。目前,对于γ-TiAl合金相变行为的研究多采用单独的水淬实验或差示扫描量热仪/差热分析仪(DSC/DTA)结合水淬实验,如Cheng和Loretto[26]通过水淬实验确定了Ti-44Al-8Nb和Ti-44Al-4Nb-4Zr-0.2Si合金的β转变温度(Tβ )和γ溶解温度(Tγ, solv)。Xu等[6]和Huang等[27]采用水淬实验的方法明确了Ti-42Al-5Mn和Ti-43.5Al-6V-1Cr合金的相变路径和凝固组织特征。另外,也有一些学者尝试采用其他实验方法,如Musi等[28]通过对Ti-44Al-(0~7)Mo合金进行原位高能X射线衍射(HEXRD)实验,获得了合金的相分数随温度的变化规律。总之,明确合金的凝固路径有助于更好地理解β凝固γ-TiAl合金复杂的微观组织演变过程,从而促进其应用与发展。
本工作以一种新型Ti-Al-Mn-Nb合金为研究对象,通过水淬实验并结合Pandat热力学计算软件,采用场发射电子探针(EPMA)、X射线衍射(XRD)、电子背散射衍射(EBSD)、透射电子显微镜(TEM)等分析方法,研究了该合金1000~1440 ℃温度范围内的相变行为,此外,对不同温度的水淬样品进行了硬度测试,研究了硬度与显微组织之间的相关性。阐明这种新型β凝固γ-TiAl合金的凝固路径和相变过程中的组织特征,以期为新型BSG合金的开发提供基础信息。
1 实验方法
以海绵Ti、工业纯Al (99.97%,质量分数)、提纯Mn、Al-Nb中间合金、TiB2粉末、石墨、高纯Si为原料,采用真空感应熔炼(VIM)制备了名义成分为Ti-43Al-1.5Mn-3Nb-0.2Si-0.2C-0.1B的新型β凝固γ-TiAl合金。合金中的O含量采用TCH600氧氮氢分析仪分析获得,Al、Mn、Nb含量采用电感耦合等离子体原子发射光谱法分析获得,最终获得合金实际成分为Ti-42.9Al-1.64Mn-2.91Nb-0.2Si-0.2C-0.1B,O含量为0.07% (质量分数)。
使用电火花线切割从铸锭中心加工若干10 mm × 10 mm × 15 mm的试样用以开展不同温度下的水淬实验。水淬实验在KSL-1700X箱式电阻炉中进行,温度为1000~1440 ℃,每间隔20 ℃设置一个实验温度。当电阻炉达到目标温度后放入样品,待电阻炉再次达到目标温度后开始计时,保温30 min后,立刻将样品取出水冷。为防止在加热过程中样品氧化对组织分析产生影响,利用电火花线切割切取水淬样品中心部分用于后续组织观察及分析。
使用JXA-iHP200F型EPMA的背散射电子(BSE)模式观察合金铸态组织和水淬样品组织。采用EPMA配备的波长色散谱(WDS)仪分析样品中各元素的分布状况和指定区域的化学成分。采用D8 Advance 型XRD分析样品相组成(Cu靶,波长为0.15406 nm,衍射角2θ范围为20°~90°,步长为5°/min)。使用配有EBSD探头的Verios 5 UC场发射扫描电子显微镜(SEM)评估样品中α、β和γ相之间的位向关系,数据结果使用AztecCrystal 2.1软件进行分析。使用Talos F200X型TEM观察片层组织形貌并获得尺寸信息。EPMA、XRD和EBSD样品制备时均首先使用SiC砂纸打磨,然后使用金刚石抛光膏和SiO2悬浮液进行粗抛和精抛。TEM样品手动打磨至60 μm后,用10%HClO4、30%C4H9OH和60%CH3OH (体积分数)的溶液在20 mA和-25 ℃环境下进行电解减薄。EBSD样品使用上述同样配比的溶液在60 V和-25 ℃环境下进行电化学抛光。使用HVS-1000Z Vickers硬度计在3 N的负载和10 s的保荷时间下测量水淬样品的显微硬度,测量10次取平均值。利用图像分析软件Image-Pro Plus 6.0统计EPMA-BSE像中β、α、γ 相的体积分数,为确保数据准确性,使用至少3张放大倍数为500的EPMA-BSE像来进行统计。
2 实验结果与分析
2.1 热力学平衡相图
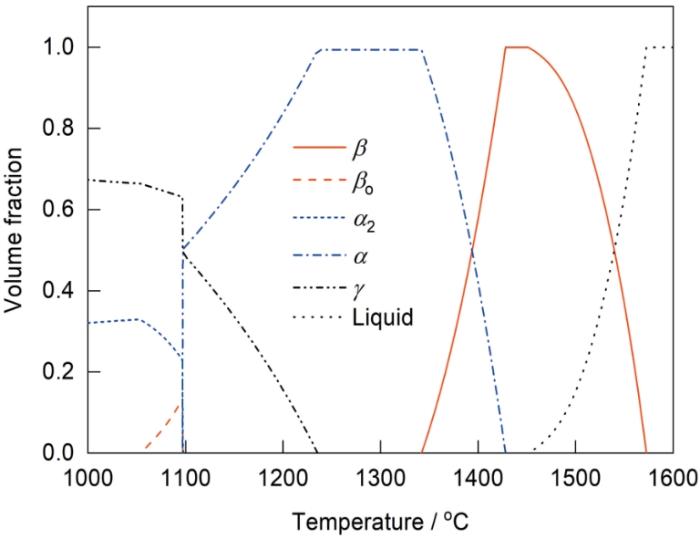
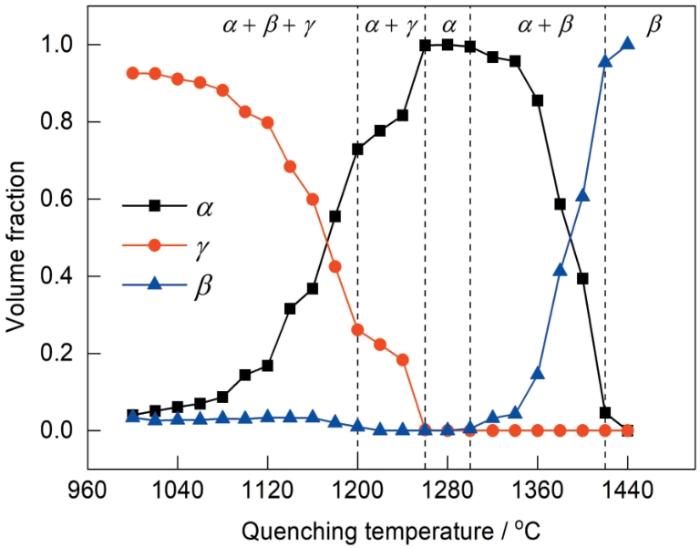
为了初步验证Ti-Al-Mn-Nb合金凝固和固态相变过程是否同时具有β和α单相区,利用Pandat软件计算了合金的热力学平衡相图(图1)。从相图中可以得到该合金在平衡条件下的凝固路径为:liquid→liquid + β→β→β + α→α→α + γ→(α2 + γ )+ βo→(α2+ γ),其中,Tβ 约为1428 ℃,Tγ,solv约为1235 ℃,共析转变温度(Teut)约为1100 ℃。热力学计算表明,本工作设计的新型合金同时具有β和α单相区,这与目前报道的Ti-42Al-5Mn、TNM等BSG合金不同,它既可以避免liquid + β→α包晶转变,具有一定的热变形能力,又可以确保合金在α单相区附近进行热处理时获得全片层组织。需要说明的是,受限于热力学数据的完善性和准确性,热力学计算相图往往难以获得合金完全准确的相变行为,特别是对于γ-TiAl合金而言,目前其相关的热力学数据库仍尚未建立完善,因此需要再次结合必要的实验手段对合金的相变路径与行为做进一步研究。
图1
2.2 铸态组织
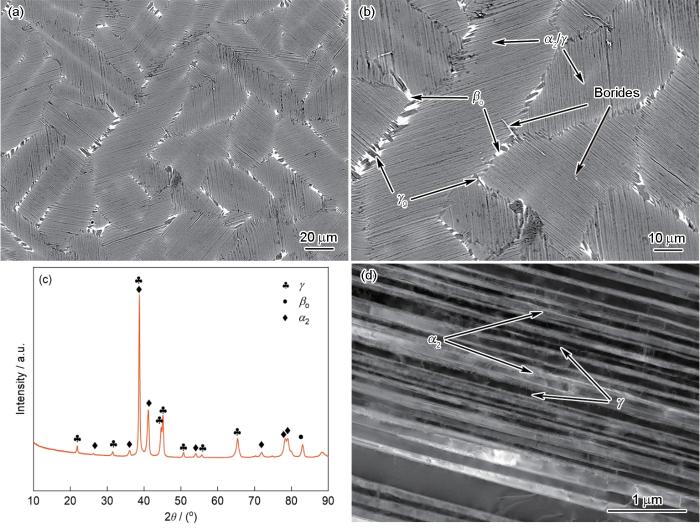
图2为新型铸态Ti-Al-Mn-Nb合金的EPMA像、XRD谱及TEM像。由EPMA像(图2a和b)和XRD谱(图2c)可知,合金基体组织主要由片层结构以及片层晶团周围的少量βo和块状γ相构成。片层内部由彼此平行、取向一致的α2-Ti3Al (D019)和γ-TiAl (L10)两相交替生长形成,平均片层间距约为55 nm (图2d),其中α2相是高温无序α相经有序化转变得到的室温稳定状态。βo-TiAl (B2)相是高温β相的室温有序状态,主要分布于片层团的边界。少量γ相呈块状镶嵌在βo相中,这种γ相又称为γ晶粒(γg)[29]。除此之外,在片层团上还可观察到呈细长条状的硼化物。γ-TiAl合金中硼化物通常以TiB2、Ti3B4及TiB的形式出现,当Al含量低于44%时,硼化物则主要以TiB为主[30]。
图2
图2
铸态Ti-Al-Mn-Nb合金的EPMA像、XRD谱及TEM像
Fig.2
Low (a) and high (b) magnified EPMA images, XRD spectrum (c), and TEM image (d) of as-cast Ti-Al-Mn-Nb alloy
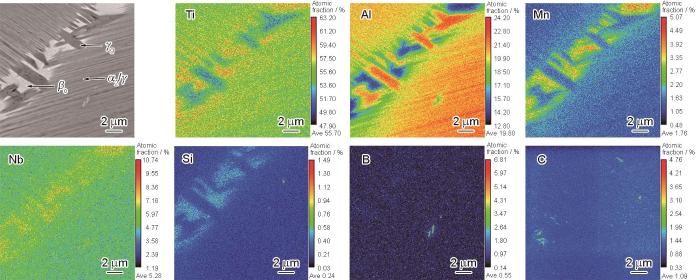
为了明确该铸态Ti-Al-Mn-Nb合金组织中不同元素在各相中的分布情况,对合金组织进行了EPMA元素面分析和点分析,结果如图3和表1所示。Ti主要富集在βo相中,在α2/γ片层中分布均匀,在γ相中负偏析。与此相反,Al主要富集在γ相中,在α2/γ片层中均匀分布,而在βo相中负偏析。Mn、Nb和Si 3种元素的分布情况相似,主要富集在βo相中,且其富集强弱顺序为Mn > Nb > Si,分别达到4.91%、3.98%和0.72%,其在γ相中的浓度略高于片层组织中。B和C主要以少量硼化物与碳化物的析出相形式存在,在α2、βo、γ 3相中并没有明显的富集现象。因此表1中各元素的原子分数并未考虑B和C 2种元素,其余元素的定量分析结果取自图3箭头所示位置。
图3
图3
铸态Ti-Al-Mn-Nb合金的EPMA像及面分析结果
Fig.3
EPMA image and element distribution maps of as-cast Ti-Al-Mn-Nb alloy
表1 图3中各相的EPMA点分析结果 (atomic fraction / %)
Table 1
| Phase | Ti | Al | Mn | Nb | Si |
|---|---|---|---|---|---|
| βo | 56.95 | 33.44 | 4.91 | 3.98 | 0.72 |
| α2/γ | 50.92 | 44.80 | 1.43 | 2.65 | 0.20 |
| γ | 49.47 | 45.28 | 1.82 | 3.11 | 0.32 |
在β凝固γ-TiAl合金中,Mn和Nb都是典型β相稳定元素。合金元素的β相稳定效应通常用每个原子的电子价态来衡量,具有更多价电子的Mn元素比Nb元素更容易实现更强的β相稳定效应[31]。同时,合金体系中原子的体积也是影响β相稳定效应的重要因素,原子半径较小的Mn元素会导致电子密度更高,单位原子体积更小,从而形成比Nb元素更稳定的β相[32],这导致Mn元素比Nb元素在βo相中更加强烈的富集。目前,无论是铸造还是锻造的γ-TiAl合金,Si、B、C已成为广泛使用的添加元素。Si作为β相的活性共析型元素,在βo相中的浓度也高于α2和γ相[33],同时Si还可以提高蠕变强度和抗氧化性[34]。而B、C能够起到细化晶粒的作用[35],进而提高合金的延展性。
2.3 水淬样品显微组织
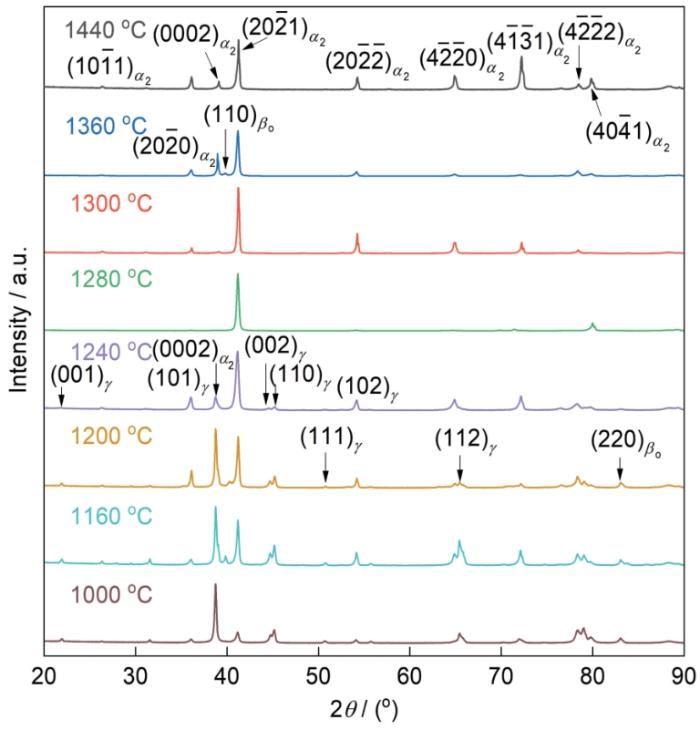
图4为Ti-Al-Mn-Nb合金在不同温度保温30 min后水淬试样的XRD谱。可以看出,在1440 ℃保温后的水淬样品中,只出现了α2的衍射峰。对于BSG合金而言,1440 ℃以上基本为单相区,这是由于高温β相水淬产生α'马氏体,Dai和Sun[36]也观察到了类似结果。由于α2和α'都是六方结构,且具有相近的晶格常数,因此很难通过XRD区分。当水淬温度降低至1360 ℃时,XRD谱中出现了βo相的衍射峰。随着水淬温度的继续降低,在1300和1280 ℃时,XRD谱中只观察到了α2相的衍射峰。当水淬温度在1240~1200 ℃之间时,XRD谱中除α2相的衍射峰以外,在1240 ℃保温后水淬样品中还出现了γ相的衍射峰。在1200 ℃时,再次出现βo相的衍射峰。在1160 ℃以下时,淬火后的合金与铸态合金的XRD谱基本保持一致,均由α2、γ和βo 3相构成。可推测在1000 ℃以下,合金中没有新的相变发生。
图4
图4
在不同温度保温30 min并水淬后Ti-Al-Mn-Nb的XRD谱
Fig.4
XRD spectra of water quenched Ti-Al-Mn-Nb alloy after holding at different temperatures for 30 min
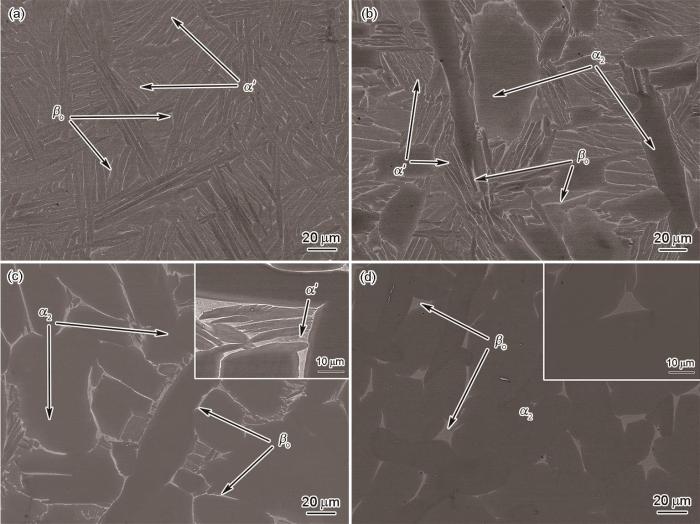
图5为Ti-Al-Mn-Nb合金在1440、1420、1360和1320 ℃保温30 min并水淬后的EPMA像。由图可知,1440 ℃保温后水淬,合金的显微组织中有序化的α'板条占主体,其板条尺寸约2.25 μm。在α'板条之间还可以观察到体积分数非常低的βo相(图5a)。1420 ℃保温后的水淬组织(图5b)中能够观察到柱状α2相从βo相中析出,表明合金在此温度下已进入α + β两相区。可以确定β→α转变的温度在1420~1440 ℃之间。当温度降低至1360和1320 ℃时,水淬样品的组织以α2相为主,同时还有少量未转变的残余βo相(图5c和d)。1360 ℃保温后的水淬样品中仍能观察到细小的板条状马氏体(图5c插图),而温度降低到1320 ℃时,水淬样品中已经观察不到马氏体(图5d插图)。
图5
图5
在1440、1420、1360和1320 ℃保温30 min并水淬后Ti-Al-Mn-Nb合金的EPMA像
Fig.5
EPMA images of water quenched Ti-Al-Mn-Nb alloy after holding at 1440 oC (a), 1420 oC (b), 1360 oC (c), and 1320 oC (d) for 30 min (Insets in Figs.5c and d show the high magnified images)
图6为Ti-Al-Mn-Nb合金在1160~1300 ℃范围内保温30 min并水淬后的EBSD像。可以看出,在1300 ℃保温后,样品组织中β→α转变基本完成,对应的显微组织在高温下以α相为主,而室温残存的βo相体积分数仅占约0.5% (图6a),因此难以通过XRD检测到βo相衍射峰(图4)。当温度降低到1280 ℃时,在图6b中只观察到α2相,结合XRD结果(图4)可以确定在1280 ℃时合金已经进入α单相区。当温度降低至1260 ℃时,α基体中开始析出少量片状γ相(图6c)。随着温度的降低,片状γ相含量逐渐增多,1240 ℃时已达到18.3% (图6d)。当温度进一步降低至1200 ℃时,α相中析出βo相,与此同时,γ相的体积分数进一步增加(图6e)。1160 ℃水淬后,开始有块状γ相在二次析出的βo相内部形成(图6f)。
图6
图6
在1300、1280、1260、1240、1200和1160 ℃保温30 min并水淬后Ti-Al-Mn-Nb合金的EPMA像
Fig.6
EPMA images of water quenched Ti-Al-Mn-Nb alloy after holding at 1300 oC (a), 1280 oC (b), 1260 oC (c), 1240 oC (d), 1200 oC (e), and 1160 oC (f) for 30 min
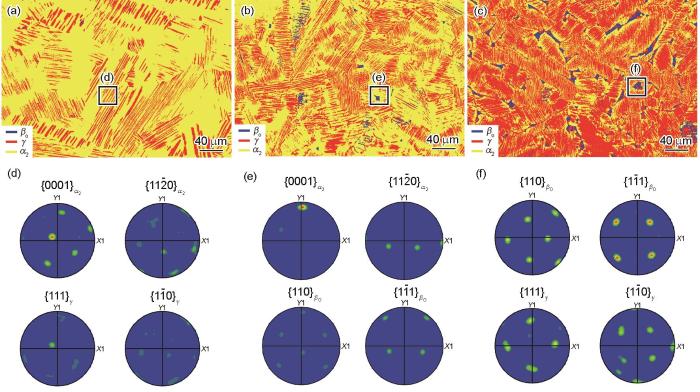
无论是CG合金还是BSG合金,都能够形成片层组织。片层组织形成的核心在于α→(α2 + γ)过程中的α→γ相变过程。如图6b和c所示,温度从1280 ℃降低至1260 ℃过程中,无序α基体中发生α→γ先共析转变从而形成片状γ相,即在γ片层预先形核阶段,来自α晶内或晶界处的Shockley不全位错将hcp结构的ABABAB堆垛次序变为fcc结构的ABCABC堆垛次序。随后通过扩散和有序化将这种fcc结构最终转变为L10结构的γ相[41]。初生γ片层形核以后,在温度降低至1240 ℃过程中,α相成分会沿着γ/α溶解度曲线变化,与此同时新的γ片层在现有γ片层的生长界面上不断形核、长大。当新生成的2个γ片层之间的α相未发生相变时,最终会形成α2/γ片层结构,如图6d和7a所示。针对α2/γ片层界面,通过对1240 ℃水淬样品进行EBSD分析,从极图(图7d)中可以发现α2/γ片层界面服从Blackburn位向关系(111) γ //(0001)
图7
图7
在不同温度下保温30 min并水淬后Ti-Al-Mn-Nb合金的EBSD相分布图和极图
Fig.7
EBSD phase maps (a-c) and pole figures (d-f) of water quenched Ti-Al-Mn-Nb alloy after holding at 1240 oC (a, d), 1200 oC (b, e), and 1160 oC (c, f) for 30 min, respectively
在Ti-Al-Mn-Nb合金凝固过程中,除主要的β→α、α→γ和α→(α2 + γ) 3种相变以外,1200 ℃保温后水淬样品中观察到βo相在α中析出(图6e和7b),这种转变称为βo析出转变。研究[45]表明,随着β→α转变的连续发生,Nb、Mn会在α相边界附近的残余β相中大量富集,当转变结束时,存在α成分偏析区域,后续的冷却过程即可发生α→βo的析出反应。在βo相的析出过程中,析出相与母相α有序化形成的α2存在Burgers位向关系(110)
图8是合金在1000~1440 ℃范围内水淬后对应组织中各相体积分数的统计结果,由于难以通过EPMA像区分β→βo和α→α2有序化转变是否发生,因此相体积分数统计时统一采用β、α和γ表示。如图所示,合金的β单相区大约在1420 ℃以上;随着温度降低到1420~1280 ℃范围内,合金进入β + α两相区;在1280~1260 ℃范围内,合金位于α单相区;在1160 ℃时合金发生共析反应,α全部转变为(α2 + γ);在1160~1000 ℃范围内,合金位于β + α + γ三相区。
图8
图8
在不同温度保温30 min并水淬后Ti-Al-Mn-Nb合金中β、α、γ相体积分数统计结果
Fig.8
Phase volume fraction results of β, α, and γ phases in water quenched Ti-Al-Mn-Nb alloy after holding at different temperatures for 30 min
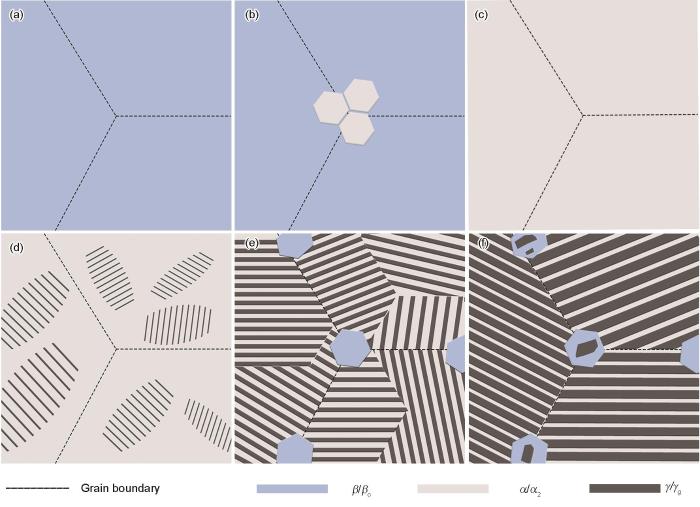
结合初生相和高温相的组成、相变过程和微观组织演变过程的结果,得出了该新型合金在冷却过程中对应的微观组织演变规律示意图,如图9所示。合金的初生相为β相(图9a),在约1420 ℃时,α相首先在β相的晶界上形核,并通过相界迁移不断生长(图9b)。在约1280 ℃时,β→α转变结束,合金进入α单相区(图9c)。随后在约1260 ℃时,开始有片层状γ相在α相中形核并长大(图9d)。随着温度不断降低,α→γ反应逐渐加剧,先共析γ相体积分数逐渐增大。值得注意的是,在β→α固相转变过程中,由于α成分偏析的存在,导致1200 ℃时再次析出βo相(图9e)。当温度降低至1160 ℃时,残余α相发生α→(α2 + γ)共析转变。块状γg的母相是二次析出的βo晶粒,因此两相的晶粒总是连续分布(图9f)。此外,水淬实验得到的凝固路径与热力学平衡相图略有差异,在实验中未观察到二次析出βo相的再次溶解。综上所述,本工作设计的新型Ti-Al-Mn-Nb合金的凝固和固态相变路径为:liquid→liquid + β→β→β + α→α→α + γ→(α2 + γ)→(α2 + γ) + βo→(α2 + γ) + βo + γg。其Tβ 、Tγ,solv和Teut分别约为1420、1280和1160 ℃。
图9
图9
Ti-Al-Mn-Nb合金在冷却过程中的相变示意图
Fig.9
Schematics illustrating the phase transformation of the Ti-Al-Mn-Nb alloy during cooling
(a) primary β phase
(b) precipitation of α from β phase
(c) transformation of the β phase into the α phase completely
(d) precipitation of γ lamellae from the α phase
(e) precipitation of βo from the lamellar colony
(f) precipitation of γg from the βo phase
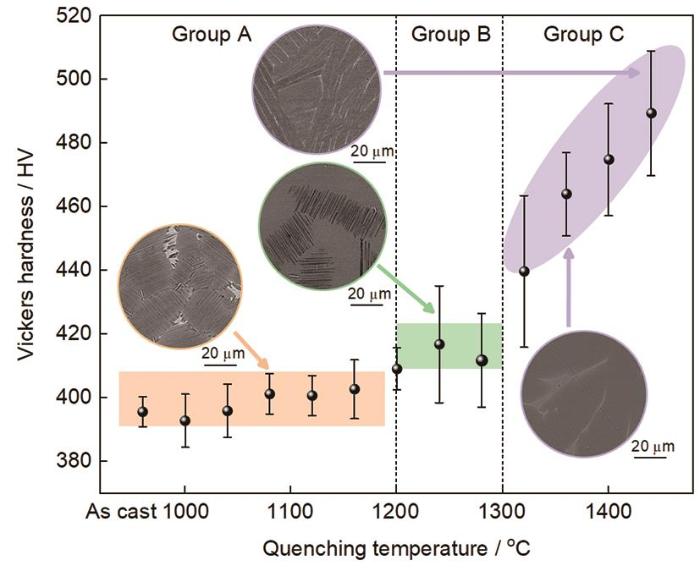
2.4 水淬样品硬度
图10
图10
在不同温度保温30 min并水淬后Ti-Al-Mn-Nb合金的Vickers硬度与显微组织
Fig.10
Correlation of Vickers hardness and water quenched microstructure of Ti-Al-Mn-Nb alloys after holding at different temperatures for 30 min
研究[50,51]表明,在TiAl合金中各相硬度的大小为:β相> α2相> γ相。对比可知,A组中合金的显微组织与铸态相似,均由α2/γ片层结构和少量βo/γ相组成。因此硬度也相近,数据波动较小,其硬度位于390~405 HV之间。从插图中可知,B组中合金的显微组织由α2相和片状γ相构成。当淬火温度达到α + γ两相区后,片层发生分解,γ相含量减少。快速冷却过程中有序化的α2相成为基体,使得组织硬度提高。1200、1240和1280 ℃水淬样品的硬度分别约为409.2、411.8和416.9 HV。C组中淬火温度已到达α + β两相区及β单相区。如插图所示,1380 ℃保温后水淬组织由α2相和βo相构成,而在1420 ℃保温后水淬组织中含有大量板条状α'与少量βo相。根据前文分析可知,随着淬火温度升高,合金组织中β相体积分数增加。同时淬火温度升高会加剧β→α'转变过程,使得具有高硬度的α'相体积分数增加。因此,C组硬度相比于A组和B组有大幅度升高。1440 ℃水淬样品硬度达到512 HV,较原始铸态组织硬度提升约23%。综上所述,新型Ti-Al-Mn-Nb合金淬火组织的硬度位于385~512 HV范围内。
3 结论
(1) 热力学平衡相图以及水淬实验结果都证实Ti-43Al-1.5Mn-3Nb-0.2Si-0.2C-0.1B合金相变过程同时具有β和α单相区,合金的凝固路径为:liquid→liquid + β→β→β + α→α→α + γ→(α2 + γ)→(α2 + γ) + βo→(α2 + γ) + βo + γg。其Tβ 、Tγ, solv和Teut分别约为1420、1280和1160 ℃。
(2) 在低于Tγ, solv温度时,合金γ相首先呈片状从α相中析出,而后发生α→α2相的有序化,且凝固过程中α2相和γ相始终满足Blackburn位向关系:(111) γ ||(0001)
(3) 合金淬火组织的Vickers硬度位于385~512 HV范围,随着淬火温度的升高淬火组织显微硬度提高,在β单相区淬火后硬度达到512 HV,较原始铸态组织硬度提升约23%。
参考文献
Administration of recombinant human granulocyte-colony stimulating factor to septic neonates induces neutrophilia and enhances the neutrophil respiratory burst and β2 integrin expression Results of a randomized controlled trial
[J].The objective of this study was to investigate the effect of treatment with recombinant human granulocyte-colony stimulating factor (rhG-CSF) on the neutrophil count and function of preterm neonates with documented sepsis. For this purpose 62 preterm neonates with proven sepsis and 19 healthy preterm ones were studied. Of the 62 patients, 27 septic neonates had an absolute neutrophil count (ANC) > 5000/mm3 (group A) and were scheduled not to receive rhG-CSF and 35/62 had an ANC < 5000/mm3 (n=35) and were randomly assigned either to receive rhG-CSF (group B) or not to receive it (group C). rhG-CSF (10 microg/kg) was administered for 3 consecutive days (0, 1, 2). The ANC, plasma levels of G-CSF (ELISA), neutrophil respiratory burst activity (NRBA) and neutrophil expression of CD11a, CD11b and CD11c (flow cytometry) were measured in all septic neonates on days 0 (onset of sepsis), 1, 3 and 5 and in the healthy neonates once within the first 2 days of life. We found that on day 0, G-CSF levels of all groups of septic neonates were significantly higher than those of the healthy ones. The highest levels were observed in group A. NRBA was diminished only in groups B and C and the expression of CD11a and CD11c was reduced in all groups of septic neonates. Administration of rhG-CSF resulted in a rapid and significant increase in ANC, NRBA and CD11a, CD11b and CD11c expression that persisted throughout the follow up. CONCLUSION; The administration of granulocyte colony stimulating factor to septic neonates significantly increases the absolute granulocyte count and enhances the neutrophil respiratory burst and beta2 integrin expression.
An overview of monolithic titanium aluminides based on Ti3Al and TiAl
[J].
Gamma titanium aluminide alloys—An assessment within the competition of aerospace structural materials
[J].
Advances in gammalloy materials-processes-application technology: Successes, dilemmas, and future
[J].
The effects of the formation of a multi-scale reinforcing phase on the microstructure evolution and mechanical properties of a Ti2AlC/TiAl alloy
[J].
Solidification pathway and phase transformation behavior in a beta-solidified gamma-TiAl based alloy
[J].The phase transformation behavior of an as-cast Ti-42Al-5 Mn (at.%) alloy after subsequent quenching from 1380 °C to 1000 °C was investigated based on the differential thermal analysis (DTA), electron probe micro analyzer-backscattered electrons (EPMA-BSE), transmission electron microscope (TEM) and X-ray diffraction (XRD). The results show that, the solidification path can be summarized as follows: Liquid→Liquid+β→β→β + α→β + α+γ→βo+α2+γ→βo+γ+α2/γ→βo+γ+α2/γ+βo,sec, with the phase transformation α→β temperature (Tβ) = 1311 °C, phase transformation γ→β temperature of (Tγsolv) = 1231 °C, phase transformation α2→α or βo→β temperature (Tα2→α/Tβo→β) = 1168 °C, eutectoid temperature (Teut) = 1132 °C and Tα2/γ→βo,sec≈1120 °C. In comparison with Ti-42Al alloy, the Teut and Tγsolv are slightly increased while both the Tβ is decreased obviously by 5% Mn addition. When quenched from the temperature of 1380-1260 °C, the martensitic transformation β→α′ could occur to form the needlelike martensite structure in β area. This kind of martensitic structure is much obvious with the increase of temperature from 1260 °C to 1380 °C. When the temperature is below Tγsolv (1231 °C), the γ grains would nucleate directly from the β phase. For the temperature slightly lower than Teut (1132 °C), the dotted βo,sec phases could nucleate in the lamellar colonies besides the γ lamellae precipitated within α2 phase. Finally, at room-temperature (RT), the alloy exhibits (βo+α2+γ) triple phase with microstructure of βo+lamellae+γ, of which the lamellar structure consists of α2, γ and βo,sec phases. The phase transformation mechanisms in this alloy, involving β→α′, β→γ, α2→α2/γ and α2→βo,sec were discussed.
Design of novel β-solidifying tial alloys with adjustable β/B2-phase fraction and excellent hot-workability
[J].
Development of β-solidifying γ-TiAl alloys sheet
[J].
Hot rolling of gamma titanium aluminide foil
[J].
Influence of processing on microstructures of Ti-44Al-llNb alloy
[J].
Effect of dwell condition on fatigue behavior of a high-Nb TiAl alloy at 750 oC
[J].
Creep behavior and related phase precipitation of a creep-resistant Y2O3-bearing high Nb containing TiAl alloy
[J].
Decomposition and phase transformation mechanisms of α2 lamellae in β-solidified γ-TiAl alloys
[J].
A review of the metastable omega phase in beta titanium alloys: The phase transformation mechanisms and its effect on mechanical properties
[J].
In-situ synchrotron HEXRD study on the phase transformation mechanisms of the ω-related phases in a Ti4Al3Nb alloy
[J].
Advances and challenges of TiAl base alloys
[J].The history of research and development of γ-TiAl intermetallic alloys was outlined and divided into 4 stages: starting (1974~1985), revolutionary (1986~1995), emerging (1996~2005) and specialty materials (2006~). Major events and landmarks at the different stages were recounted to provide a framework for understanding scientific and technological progress. Key advances in the following 6 areas were reviewed: alloying, microstructure type, primary processing (melting), secondary processing (hot working), properties (including creep, fracture and fatigue, and oxidation), and tertiary processing (forming, covering both investment casting and near-net shape powder metallurgy). Future challenges were identified as follows: improvement of centrifugal casting technology, low-cost wrought process, development of third-generation alloys that meet design specifications, new applications based on new technologies, and viability of new forming routes such as additive manufacturing。
钛铝金属间化合物的进展与挑战
[J].
Phase equilibria among α (hcp), β (bcc) and γ (L10) phases in Ti-Al base ternary alloys
[J].
Development of gamma titanium aluminide (γ-TiAl) alloys: A review
[J].
History and development of γ-TiAl alloys and the effect of alloying elements on their phase transformations
[J].
Effects of trace alloying elements on the phase transformation behaviors of ordered ω phases in high Nb-TiAl alloys
[J].
Cyclic oxidation behavior of Nb/Mn/Si alloying beta-gamma TiAl alloys
[J].
Improved high-temperature oxidation properties for Mn-containing beta-gamma TiAl with W addition
[J].
Design, processing, microstructure, properties, and applications of advanced intermetallic TiAl alloys
[J].
Microstructural evolution and mechanical properties of forged β-solidified γ-TiAl alloy by different heat treatments
[J].
Reassessment of the binary aluminum-titanium phase diagram
[J].
The decomposition of the beta phase in Ti-44Al-8Nb and Ti-44Al-4Nb-4Zr-0.2Si alloys
[J].
Phase transformation and microstructure evolution of a beta-solidified gamma-TiAl alloy
[J].
Phase transformations and phase stability in the Ti-44 at.% Al-(0-7 at.%) Mo system
[J].
Evolution of the ωo phase in a β-stabilized multi-phase TiAl alloy and its effect on hardness
[J].
Structural stability and the alloying effect of TiB polymorphs in TiAl alloys
[J].
First-principles study of phase stability and elastic properties of binary Ti-xTM (TM = V, Cr, Nb, Mo) and ternary Ti-15TM-yAl alloys
[J].
Phase stability of TiAl-X (X = V, Nb, Ta, Cr, Mo, W, and Mn) alloys
[J].
Precipitates and alloying elements distribution in near α titanium alloy Ti65
[J].Precipitates, including silicides and Ti3Al (α2) phase, and alloying elements distribution in a near α titanium alloy Ti65 (Ti-5.8Al-4.0Sn-3.5Zr-0.5Mo-0.3Nb- 1.0Ta-0.4Si-0.8W-0.05C) after solution treatment and aging process were characterized by using transmission electron microscopy (TEM) and atom probe tomography (APT). Quantitative composition analysis and TEM observation indicate that the silicides fit to (Ti, Zr)6(Si, Sn)3. Zr exhibits a β-stabilizing effect in near α titanium alloys but is weaker than other β stabilizing elements. The enriching tendency of the alloying elements in the retained β phase is in the order of Zr < Nb < Ta < Mo < W. The experimental results are rationalized by the relative stability of alloying elements in the α and β phases and the mobility of these atoms in the matrix. An enrichment of Si in the α2 phase over the α matrix phase is noticed, which is attributed to the lower formation energy of Si in the α2 phase.
The Ti3(Al, Si) + Ti5(Si, Al)3 eutectic reaction in the Ti-Al-Si system
[J].
Effects of tantalum on microstructure evolution and mechanical properties of high-Nb TiAl alloys reinforced by Ti2AlC
[J].
Microstructure optimization and improved tensile property in a high Nb-containing γ-TiAl alloy
[J].
Phase transformation behavior of a β-solidifying γ-TiAl-based alloy from different phase regions with various cooling methods
[J].
Phase transformations in a β-solidifying γ-TiAl based alloy during rapid solidification
[J].
Phase transformations and modulated microstructures in Ti-Al-Nb alloys
[J].
Multi-twinned deformation and fracture characteristics of directional solidified Ti-45.5Al-5Nb-0.5Ta alloys during high-temperature rotary-bending fatigue process
[J].
Weak-beam observation of a dissociation transition in TiAl
[J].
Phase transformation mechanisms involved in two-phase TiAl-based alloys—I. Lambellar structure formation
[J].
Phase transformations in γ based titanium aluminides
[J].
Atomic-scale understanding of the γ/α2 interface in a TiAl alloy
[J].
Microsegregation in high Nb containing TiAl alloy ingots beyond laboratory scale
[J].
Strengthening behavior of beta phase in lamellar microstructure of TiAl alloys
[J].
Intermetallic β-solidifying γ-TiAl based alloys—From fundamental research to application
[J].
Study on the microstructure, phase transition and hardness for the TiAl-Nb alloy design during directional solidification
[J].
Effect of cooling rate on phase transformation in Ti2AlNb alloy
[J].
High tensile ductility and strength in the Ti-42Al-6V-1Cr alloy
[J].
Microstructure development and hardness of a powder metallurgical multi phase γ-TiAl based alloy
[J].