2020年,国家明确提出“碳达峰”和“碳中和”的双碳战略,降低碳排放已经深入到各行各业。钢铁行业是碳排放量最高的工业门类,降低钢材损耗有利于降低钢铁产业的碳排放。腐蚀是工业设施的慢性病,每年约30%的钢铁产品由于腐蚀而发生报废,钢材腐蚀不仅关系到经济、安全问题,同时也影响双碳战略的施行。镀层是一种有效提高钢的耐腐蚀性能的方法,目前应用较为广泛的镀锌层随着服役环境的变化,面临着镀层过厚、耐蚀性不足等问题。Zn-Al-Mg镀层是在镀锌的基础上添加少量的Al、Mg元素制成的,其耐腐蚀性能优异,为镀锌产品的5倍以上[1],同时具有优异的切边保护性、加工性、耐磨性等特点,在汽车、光伏发电、建筑等领域均有着广泛的应用前景[1~17],是恶劣环境下替代镀锌的优良材料。
根据添加的Al含量不同,可以将Zn-Al-Mg镀层分为“低铝”(Al质量分数wAl ≤ 5%)、“中铝”(5% < wAl < 13%)和“高铝”(47% < wAl < 57%) 3种类型。本工作的Zn-2.0Al-1.5Mg镀层为低铝型Zn-Al-Mg镀层,低铝型Zn-Al-Mg镀层具有良好的耐蚀性、成形性和焊接性,在家电及汽车等行业应用广泛。Al、Mg含量会对镀层中组织产生一定影响[18]。一般Zn-Al-Mg镀层由纯Zn相或纯Al相、Zn/MgZn2二元共晶相、Zn/MgZn2/Al三元共晶相、MgZn2相和Mg2Si相中的几种构成[9,19~22]。低铝Zn-Al-Mg镀层含有纯Zn相、Zn/MgZn2二元共晶相、Zn/MgZn2/Al三元共晶相3种组织。有研究[23]指出,共晶组织增多,腐蚀失重有减小的趋势。科研人员[19,20,24~26]利用原位监测发现二元共晶相中的MgZn2相会首先被腐蚀,Thierry等[27]提出,Zn-Al-Mg镀层会在共晶相处优先发生腐蚀。同时,Mg和Al的加入能够抑制ZnO的生成,促进保护性的腐蚀产物Zn5(OH)8Cl2·H2O、Zn4(SO4)(OH)6·nH2O等的形成[28~32]。
镀层在实际大气环境中服役而引发的大气腐蚀,是一个材料与环境之间相互作用的复杂过程,根据实际大气环境情况分为工业大气腐蚀、海洋大气腐蚀、城市大气腐蚀、乡村大气腐蚀等,其中海洋大气腐蚀较为严重,腐蚀等级甚至能够达到最高的CX级[33,34],因此对于服役于海洋大气环境中的材料来说其耐腐蚀性能值得重点关注,如具有较好耐腐蚀性能的Zn-Al-Mg镀层。然而,Zn-Al-Mg镀层问世时间较短,在海洋大气这种高腐蚀环境下服役情况有待进一步研究。本工作利用Q-FOG盐雾实验箱,以海洋大气中主要成分NaCl为腐蚀介质,设计了一种模拟海洋大气环境,采用失重分析、扫描电镜(SEM)、X射线衍射(XRD)分析及电化学测试等方法研究Zn-2.0Al-1.5Mg镀层在模拟海洋大气中的腐蚀行为及作用机理,以期为Zn-2.0Al-1.5Mg镀层服役于海洋大气环境积累腐蚀数据,并为Zn-2.0Al-1.5Mg镀层优化提供数据参考。
1 实验方法
实验所用材料为Zn-2.0Al-1.5Mg (质量分数,%)镀层,镀层质量为275 g/m2,镀层均匀,厚度约为20 μm。试样尺寸为100 mm × 50 mm × 0.85 mm。实验前样品表面均经过酒精和丙酮清洗,为避免边缘效应,样品均经过封边处理。
实验在Q-FOG型盐雾试验箱中进行,腐蚀介质选用5%NaCl (质量分数),采用如下循环加速程序来模拟海洋大气环境:每个循环12 h (1. 喷盐2 h,35 ℃;2. 干燥4 h,35 ℃;3. 湿润1 h,35 ℃;4. 干燥2 h,60 ℃;5. 重复步骤3和4一次),取样周期分别为168、336、504、672、840和1848 h。
每周期取3个平行样品用于腐蚀失重分析,以确定Zn-2.0Al-1.5Mg镀层的腐蚀速率。依据ISO 8407:2009,Zn-2.0Al-1.5Mg镀层暴晒后去除腐蚀产物的方法如下:首先去除表面疏松的腐蚀产物,之后在室温下,将样品置于饱和甘氨酸溶液1~10 min,去除余下腐蚀产物,最后将样品用去离子水冲洗,用酒精脱水并烘干。在干燥箱中干燥24 h后,用分析天平称量确定腐蚀失重。
采用Nikon D50数码相机观察样品表面的宏观形貌。采用ESEM XL30 FEG型SEM和Inca X-Max型能谱仪(EDS)对腐蚀产物的表面、截面形貌和成分进行观察和分析。截面形貌样品嵌入环氧树脂中密封后,用SiC砂纸打磨至2000号,利用金刚石抛光膏(2.5 μm)抛光,之后使用去离子水和酒精进行清洁吹干。样品拍摄前均进行喷碳处理。
采用XPERT-PRO XRD对腐蚀产物进行鉴定,Cu靶,电压40 kV,电流40 mA,扫描范围4°~90°。扫描速率4°/min。
利用PARSTAT 2273电化学工作站进行电化学阻抗谱(EIS)和动电位极化测量。每周期设置3组平行样,测试面积为1 cm2。测试在盛有0.1 mol/L NaCl溶液的三电极电解槽中进行,Pt电极为对电极,饱和甘汞电极为参比电极,腐蚀试样为工作电极。测试前需确保电位稳定。EIS测试参数选用振幅为10 mV的正弦波,频率范围10 mHz~100 kHz。动电位极化测试的扫描速率为0.3333 mV/s,扫描范围为-0.25~0.3 V (vs OCP (开路电位))。
2 实验结果
2.1 腐蚀动力学
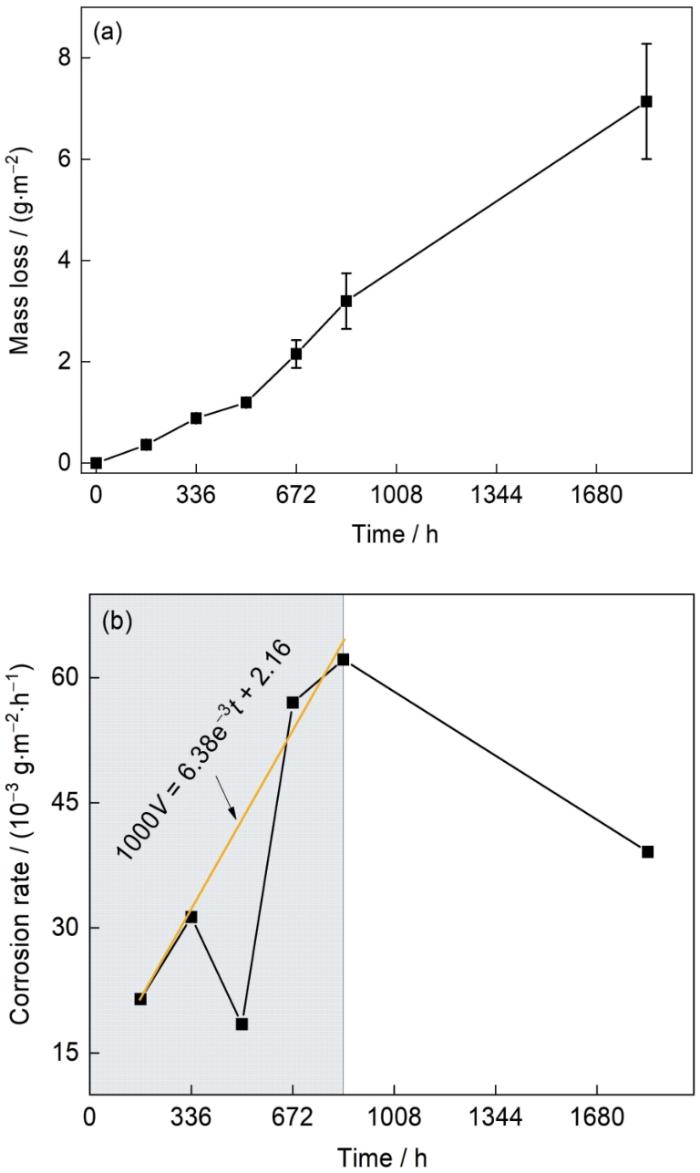
图1
图1
Zn-2.0Al-1.5Mg镀层在模拟海洋大气环境中腐蚀失重和平均腐蚀速率随腐蚀时间的变化
Fig.1
Mass losses (a) and average corrosion rates (b) of Zn-2.0Al-1.5Mg coating in the simulated marine atmosphere at different time (V—corrosion rate, t—corrosion time)
式中,V为腐蚀速率(g/(m2·h)),t为时间(h),w为单位面积腐蚀失重(g/m2),下标n为周期数。由图1b可见,腐蚀速率的波动呈M型,在0~840 h,除336~504 h的腐蚀速率出现减小外,其余周期腐蚀速率随时间延长而增加,基本呈现线性规律:
在840~1848 h阶段,其腐蚀速率下降,不再遵循线性增加的腐蚀规律。
2.2 腐蚀产物组成
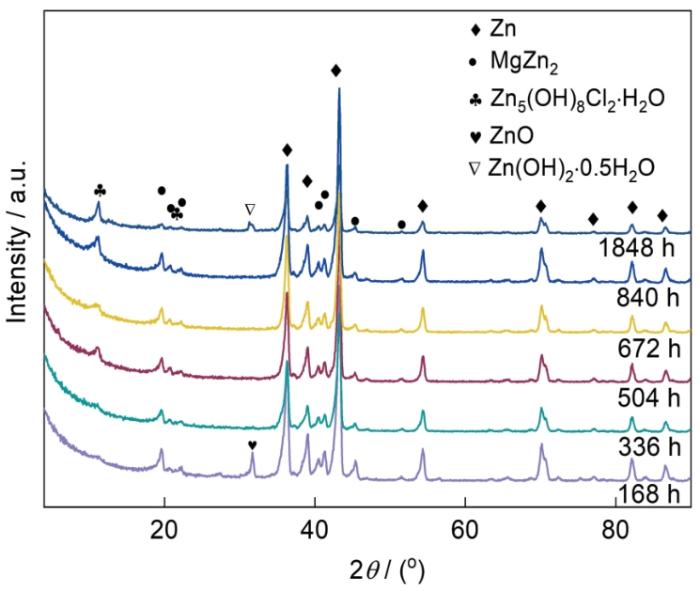
图2
图2
Zn-2.0Al-1.5Mg镀层腐蚀不同时间的XRD谱
Fig.2
XRD spectra of Zn-2.0Al-1.5Mg coating in the simulated marine atmosphere at different time
2.3 腐蚀产物形貌
2.3.1 宏观形貌
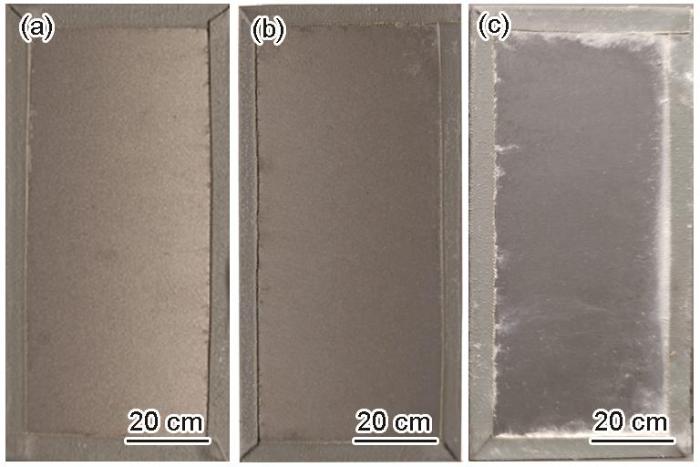
图3为Zn-2.0Al-1.5Mg镀层在模拟海洋大气环境中腐蚀168、840和1848 h后的宏观形貌。在0~840 h内,腐蚀较轻,样品表面光泽度减小,颜色加深,未发现明显的白色腐蚀产物。腐蚀1848 h,白色腐蚀产物集中在封边胶带附近,样品中部的白色腐蚀产物并不明显。
图3
图3
Zn-2.0Al-1.5Mg镀层在模拟海洋大气环境中腐蚀不同时间的宏观形貌
Fig.3
Macromorphologies of Zn-2.0Al-1.5Mg coating in the simulated marine atmosphere at different time
(a) 168 h (b) 840 h (c) 1848 h
2.3.2 微观形貌
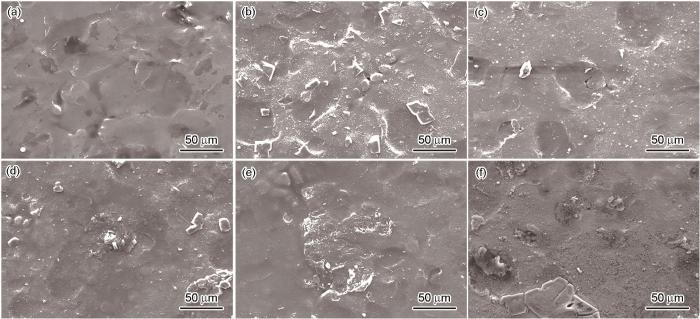
图4
图4
Zn-2.0Al-1.5Mg镀层在模拟海洋大气环境中腐蚀不同时间后表面形貌的SEM像
Fig.4
Surface SEM images of Zn-2.0Al-1.5Mg coating in the simulated marine atmosphere at different time
(a) 168 h (b) 336 h (c) 504 h (d) 672 h (e) 840 h (f) 1848 h
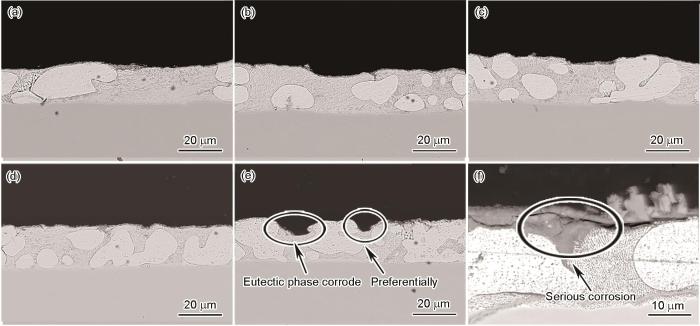
图5为Zn-2.0Al-1.5Mg镀层在模拟海洋大气环境中腐蚀不同时间后截面形貌的SEM像。可以看出,在0~672 h内,腐蚀程度较轻;腐蚀840 h,在共晶相处优先腐蚀;至1848 h后,腐蚀严重,镀层表面有明显的腐蚀产物覆盖。在靠近纯Zn相的共晶相处腐蚀最为严重,腐蚀产物层有裂纹产生。
图5
图5
Zn-2.0Al-1.5Mg镀层在模拟海洋大气环境中腐蚀不同时间后截面形貌的SEM像
Fig.5
Cross-sectional SEM images of Zn-2.0Al-1.5Mg coating in the simulated marine atmosphere at different time
(a) 168 h (b) 336 h (c) 504 h (d) 672 h (e) 840 h (f) 1848 h
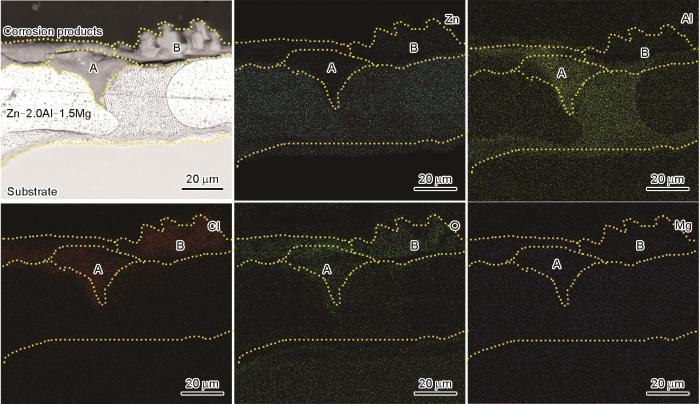
图6
图6
Zn-2.0Al-1.5Mg镀层在模拟海洋大气环境中腐蚀1848 h后截面形貌的SEM像和EDS元素分布
Fig.6
SEM image and corresponding EDS mappings of elements of Zn-2.0Al-1.5Mg coating in the simulated marine atmosphere at 1848 h
2.4 电化学分析
为进一步研究Zn-2.0Al-1.5Mg镀层在模拟海洋大气中腐蚀行为的变化,对腐蚀不同周期的样品进行电化学分析。电化学测试反映的腐蚀速率是瞬时的腐蚀速率,而腐蚀失重反映的腐蚀速率是平均腐蚀速率。如电化学测试中0 h对应的值为原始未腐蚀样品的测试值,其耐腐蚀性能的优劣直接影响0~168 h的腐蚀速率,因此电化学测试中0 h对应腐蚀失重0~168 h的平均腐蚀速率,即图1b中168 h所对应的点。而电化学测试中1848 h的结果预示着进一步腐蚀的腐蚀速率。
2.4.1 动电位极化结果
图7
图7
Zn-2.0Al-1.5Mg镀层腐蚀不同时间的动电位极化曲线
Fig.7
Potentiodynamic curves of Zn-2.0Al-1.5Mg coating in the simulated marine atmosphere at different time (E—potential, i—current density, SCE—saturated calomel electrode)
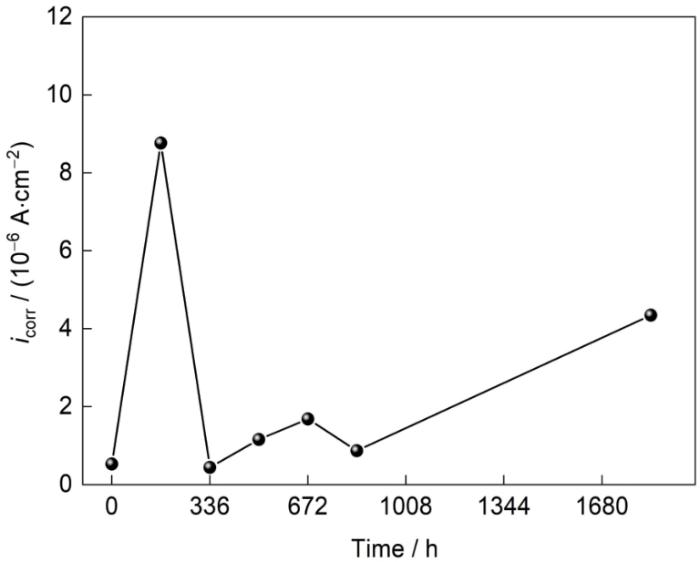
图8
图8
Zn-2.0Al-1.5Mg镀层腐蚀不同时间的腐蚀电流密度(icorr)
Fig.8
Corrosion current density (icorr) of Zn-2.0Al-1.5Mg coating obtains by Tafel extrapolation after different exposure periods
2.4.2 EIS
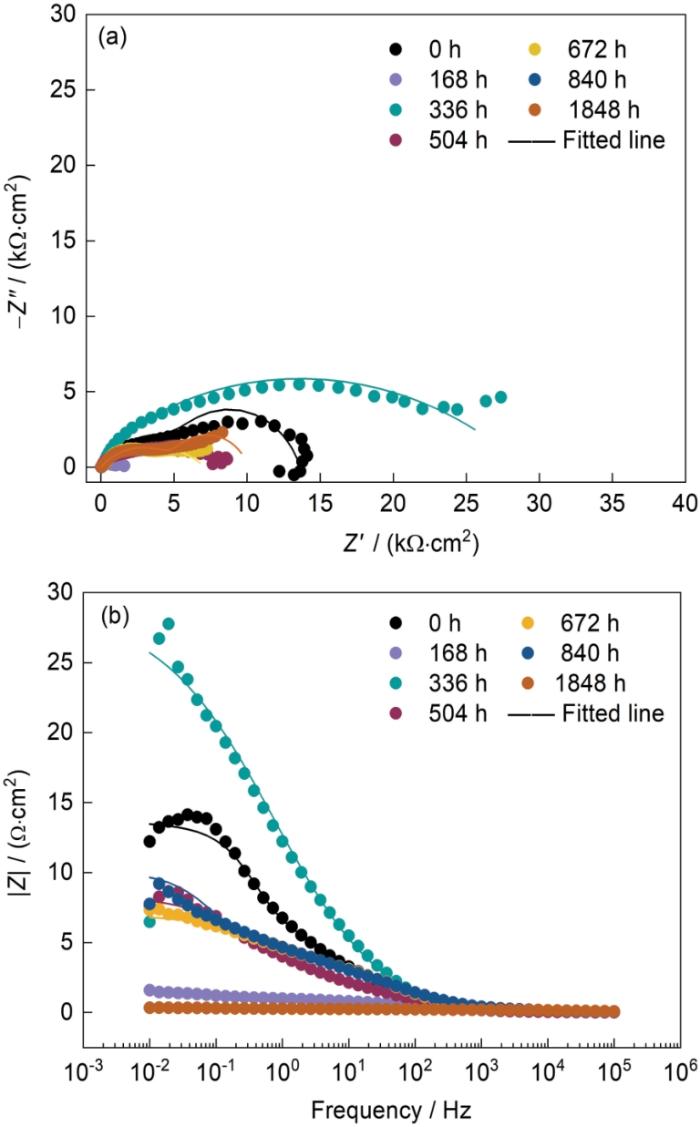
图9为Zn-2.0Al-1.5Mg镀层在模拟海洋大气中腐蚀不同时间的EIS结果。一般地,腐蚀速率与极化电阻(Rp)成反比,Rp可表示为:
图9
图9
Zn-2.0Al-1.5Mg镀层腐蚀不同时间的Nyquist和Bode图
Fig.9
Nyquist (a) and Bode (b) diagrams of Zn-2.0Al-1.5Mg coating in the simulated marine atmosphere at different time (Z'—impedance real part, Z"—impedance imaginary part, Z—impedance)
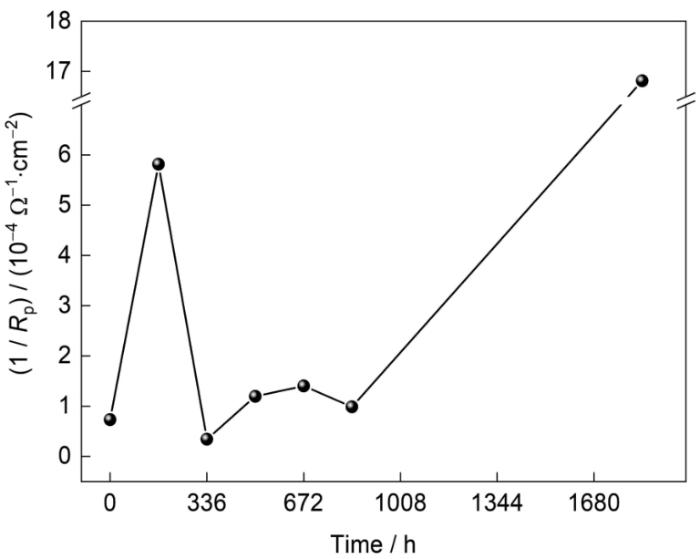
式中,|ZL|和|ZH|分别为低频(10 mHz)和高频(10 kHz)阻抗。如图9b所示,腐蚀336 h样品最耐蚀,优于未腐蚀的原始镀层(0 h)。腐蚀504、672和840 h的样品的耐腐蚀性能相差不大,而腐蚀168和1848 h的样品的耐腐蚀性能相对较差。
图10
图10
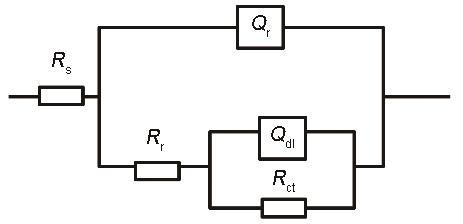
电化学阻抗谱(EIS)等效电路
Fig.10
Equivalent circuit of EIS (Rs—electrolyte resistance, Rr—rust layer resistance, Rct—charge transfer resistance, Qr—rust layer capacitance, Qdl—double layer capacitance, EIS—electrical impedance spectroscopy)
表1 EIS参数拟合结果
Table 1
Time h | Rs Ω·cm2 | Qr | Rr Ω·cm2 | Qdl | Rct Ω·cm2 | Chi-squared | Rp (= Rr + Rct) Ω·cm2 | ||
|---|---|---|---|---|---|---|---|---|---|
yr Ω-1·cm-2·S | nr | ydl Ω-1·cm-2·S | ndl | ||||||
| 0 | 4.33 × 101 | 1.30 × 10-5 | 6.56 × 10-1 | 7.20 × 103 | 6.26 × 10-5 | 9.71 × 10-1 | 6.44 × 103 | 9.29 × 10-3 | 1.36 × 104 |
| 168 | 5.05 × 101 | 1.48 × 10-5 | 6.81 × 10-1 | 8.26 × 102 | 2.05 × 10-3 | 5.23 × 10-1 | 8.94 × 102 | 5.87 × 10-3 | 1.72 × 103 |
| 336 | 8.53 × 10-4 | 2.02 × 10-5 | 4.65 × 10-1 | 2.31 × 102 | 6.74 × 10-7 | 9.60 × 10-1 | 2.87 × 104 | 2.39 × 10-2 | 2.89 × 104 |
| 504 | 2.51 × 101 | 1.07 × 10-5 | 6.95 × 10-1 | 2.84 × 103 | 1.19 × 10-4 | 6.36 × 10-1 | 5.52 × 103 | 7.11 × 10-3 | 8.36 × 103 |
| 672 | 4.21 × 10-3 | 3.03 × 10-5 | 4.58 × 10-1 | 7.89 × 102 | 2.09 × 10-7 | 1.00 × 100 | 6.33 × 103 | 6.47 × 10-3 | 7.12 × 103 |
| 840 | 3.07 × 101 | 1.77 × 10-5 | 5.50 × 10-1 | 5.52 × 103 | 4.13 × 10-4 | 8.78 × 10-1 | 4.59 × 103 | 4.34 × 10-2 | 1.01 × 104 |
| 1848 | 3.14 × 10-23 | 1.51 × 10-3 | 8.38 × 10-2 | 4.70 × 10-19 | 4.63 × 10-7 | 7.34 × 10-1 | 5.95 × 102 | 7.36 × 10-4 | 5.95 × 102 |
图11
图11
1/ Rp随腐蚀时间的变化
Fig.11
Variations of 1/ Rp after different corrosion periods
2.5 腐蚀机理
Zn-2.0Al-1.5Mg镀层在模拟海洋大气环境下发生腐蚀,腐蚀速率呈M型变化,0~840 h内腐蚀速率主体呈上升趋势。依据XRD谱结果,腐蚀168 h,主要的腐蚀产物为ZnO,形成过程如式(
值得注意的是,腐蚀失重结果中,腐蚀速率虽呈M型变化规律,但是整体呈上升趋势,只在336~504 h出现下降。电化学结果所反映出来的是168 h腐蚀速率的急剧升高,336 h的腐蚀速率下降,0、504和672 h呈现上升趋势。2者反映结果的差异性主要集中在168 h时,其与所处的环境有密切关系。168 h腐蚀速率剧烈上升的原因可能是电化学测试环境为NaCl溶液,不再是干燥时间较长的模拟海洋大气环境,当干燥环境下腐蚀168 h的样品的ZnO腐蚀产物置于湿润环境中,ZnO进一步发生反应,加速反应进程,提高腐蚀速率。这使得电化学结果中,168 h的样品具有较差的耐腐蚀性能。
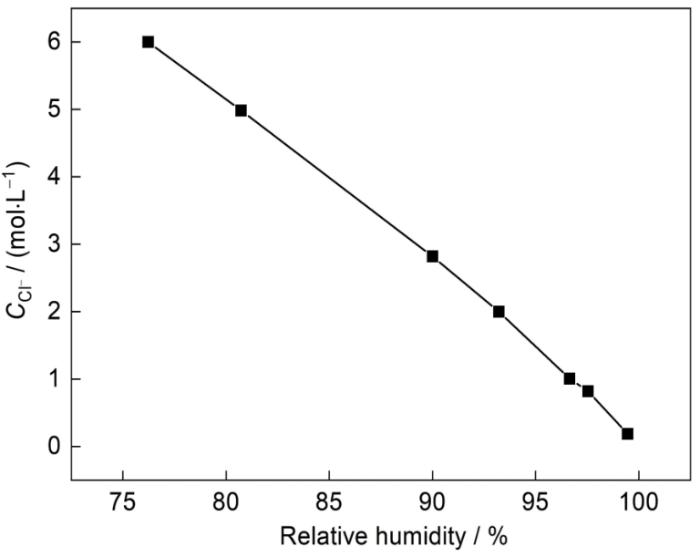
大气腐蚀是发生在薄液膜下的电化学过程,薄液膜的体积随着湿度的变化而发生变化,当体积变化时,液膜中腐蚀离子的浓度随之改变。同时腐蚀介质只有在湿度达到潮解湿度(DRH)时才会在金属表面溶解成电解质,而湿度为风化湿度(ERH)时,电解质才能结晶。因此湿度在大气腐蚀的反应过程中发挥着重要作用。以NaCl为例,当其DRH为75%时,ERH为41%~51%。Cl-的平衡浓度(
图12
与作者前期工作[39]相比,本工作模拟海洋大气环境下含Cl的腐蚀产物Zn5(OH)8Cl2·H2O较少,主要与高温干燥阶段缩短了干湿交替时间和Cl-反应时间密切相关。腐蚀至1878 h,有Zn(OH)2·0.5H2O生成,这是由于随着腐蚀时间的延长,MgZn2被消耗,少量的Mg难以发挥作用,使得Zn(OH)2·0.5H2O在后期腐蚀阶段生成。同时根据元素分析,Al元素也在一定程度上影响了腐蚀产物Zn(OH)2·0.5H2O的生成。
3 结论
(1) Zn-2.0Al-1.5Mg镀层在模拟海洋大气中腐蚀168 h,腐蚀产物为ZnO;随着腐蚀时间的延长,腐蚀产物主要为Zn5(OH)8Cl2·H2O;到1848 h,腐蚀产物中还有少量的Zn(OH)2·0.5H2O。ZnO出现的主要原因之一是干湿交替时间缩短,而Zn(OH)2·0.5H2O的出现与腐蚀后期Mg或Al元素的消耗有关。
(2) 在模拟海洋大气中,Zn-2.0Al-1.5Mg镀层腐蚀速率随时间呈M型变化,840 h前大体呈上升趋势,仅在336~504 h 阶段腐蚀速率减小,其原因可能与腐蚀产物ZnO的消失和Zn5(OH)8Cl2·H2O的比例增多有关。结合电化学结果推断1848 h后进一步腐蚀,腐蚀可能有加快趋势。
参考文献
Development of hot dip galvanized aluminum and magnesium coated plate and application status of Hegang and Tangshan Steel
[J].
热镀锌铝镁镀层板发展及河钢唐钢应用现状
[J].
Improving corrosion stability of Zn-Al-Mg by alloying for protection of car bodies
[J].
Coil-coated Zn-Mg and Zn-Al-Mg: Effect of climatic parameters on the corrosion at cut edges
[J].
Corrosion performance of Zn-Mg-Al coated steel in accelerated corrosion tests used in the automotive industry and field exposures
[J].
Genesis and mechanism of microstructural scale deformation and cracking in ZnAlMg coatings
[J].
MagiZinc—The new high performance coating for steel in the BIW and closures
[A].
Chemistry of corrosion products on Zn-Al-Mg alloy coated steel
[J].
Corrosion performance of Zn-Al-Mg coatings in open and confined zones in conditions simulating automotive applications
[J].
Long-term atmospheric corrosion rates of hot dip galvanised steel and zinc-aluminium-magnesium coated steel
[J].
The effect of sulphate, phosphate, nitrate and acetate on the corrosion behaviour of Zn-Al-Mg hot-dip galvanised steel
[J].
Corrosion of wire arc sprayed ZnMgAl
[J].
Morphology and properties of hot dip Zn-Mg and Zn-Mg-Al alloy coatings on steel sheet
[J].
Effects of Mg content on microstructure and electrochemical properties of Zn-Al-Mg alloys
[J].
Corrosion behaviour of Zn-Al-Mg coated steel sheet in sodium chloride-containing environment
[J].
Electrochemical monitoring of the degradation of galvanized steel in simulated marine atmosphere
[J].Galvanized steels with different thicknesses of coatings were exposed to the wet-dry cycles in the presence of NaCl. The degradation of galvanized steels was investigated at different exposure times by electrochemical impedance spectroscopy. Further, the degradation is categorized into four characteristic stages, including tau(1): dissolution of zinc, tau(2): galvanic protection by metallic zinc, tau(3): barrier protection by zinc corrosion products, and tau(4): onset of the underlying steel corrosion, respectively. The underlying steel was protected by the zinc corrosion products for a relatively long time even after the metallic zinc coating was completely corroded (i.e., the tau(3) stage).
Atmospheric corrosion of Galfan coatings on steel in chloride-rich environments
[J].
A critical review on corrosion and runoff from zinc and zinc-based alloys in atmospheric environments
[J].
Development and application of hot-dip galvanized zinc-aluminum-magnesium coating
[J].
热浸镀锌铝镁镀层开发及应用进展
[J].过去20年,国际上成功商业化了热浸镀锌铝镁镀层。该镀层以Zn、Al、Mg三元合金为主,按Al含量的范围,可分成“低铝”、“中铝”、“高铝”3大类。因锌铝镁镀层具有高平面耐蚀性和高切口耐蚀性的特点,在建筑业和家电业得到了广泛的应用。同时锌铝镁镀层还具有摩擦因数低且稳定、以及镀层冲压磨损量少等特性,因此近年来在汽车工业的应用也正在逐渐拓展。简要回顾了锌铝镁镀层的发展历程,重点围绕镀层成分、组织、性能、应用等方面进行了介绍,并探讨了锌铝镁镀层未来发展趋势。
In situ monitoring of corrosion mechanisms and phosphate inhibitor surface deposition during corrosion of zinc-magnesium-aluminium (ZMA) alloys using novel time-lapse microscopy
[J].In situ time-lapse optical microscopy was used to examine the microstructural corrosion mechanisms in three zinc-magnesium-aluminium (ZMA) alloy coated steels immersed in 1% NaCl pH 7. Preferential corrosion of MgZn(2) lamellae within the eutectic phases was observed in all the ZMA alloys followed by subsequent dissolution of Zn rich phases. The total extent and rate of corrosion, measured using time-lapse image analysis and scanning vibrating electrode technique (SVET) estimated mass loss, decreased as Mg and Al alloying additions were increased up to a level of 3 wt% Mg and 3.7 wt% Al. This was probably due to the increased presence of MgO and Al(2)O(3) at the alloy surface retarding the kinetics of cathodic oxygen reduction. The addition of 1 × 10(-2) mol dm(-3) Na(3)PO(4) to 1% NaCl pH 7 had a dramatic influence on the corrosion mechanism for a ZMA with passivation of anodic sites through phosphate precipitation observed using time-lapse image analysis. Intriguing rapid precipitation of filamentous phosphate was also observed and it is postulated that these filaments nucleate and grow due to super saturation effects. Polarisation experiments showed that the addition of 1 × 10(-2) mol dm(-3) Na(3)PO(4) to the 1% NaCl electrolyte promoted an anodic shift of 50 mV in open circuit potential for the ZMA alloy with a reduction in anodic current of 2.5 orders of magnitude suggesting that it was acting primarily as an anodic inhibitor supporting the inferences from the time-lapse investigations. These phosphate additions resulted in a 98% reduction in estimated mass loss as measured by SVET demonstrating the effectiveness of phosphate inhibitors for this alloy system.
In situ monitoring of the microstructural corrosion mechanisms of zinc-magnesium-aluminium alloys using time lapse microscopy
[J].
The corrosion behavior for Zn-Al-Mg coating in NaCl system
[J].
锌铝镁镀层在NaCl体系中的腐蚀行为
[J].
Surface and cut-edge corrosion behavior of Zn-Mg-Al Alloy-coated steel sheets as a function of the alloy coating microstructure
[J].
Advances in continuous hot dip galvanizing of advanced high strength steels
[A].
先进高强钢连续热浸镀锌铝镁技术的研究进展
[A].
Initial SO2-induced atmospheric corrosion of ZnAlMg coated steel studied with in situ Infrared Reflection Absorption Spectroscopy
[J].
Effect of the microstructure of Zn-Al and Zn-Al-Mg model alloys on corrosion stability
[J].
Corrosion mechanisms of Zn(Mg,Al) coated steel: The effect of HCO
Atmospheric corrosion of ZnAlMg coated steel during long term atmospheric weathering at different worldwide exposure sites
[J].
Corrosion mechanisms of Zn(Mg, Al) coated steel: 2. The effect of Mg and Al alloying on the formation and properties of corrosion products in different electrolytes
[J].
Understanding corrosion via corrosion product characterization: II. Role of alloying elements in improving the corrosion resistance of Zn-Al-Mg coatings on steel
[J].
Corrosion mechanisms of Zn(Mg, Al) coated steel in accelerated tests and natural exposure: 1. The role of electrolyte composition in the nature of corrosion products and relative corrosion rate
[J].
Characterization of corrosion products of Zn and Zn-Mg-Al coated steel in a marine atmosphere
[J].
Research on ZnAlMg coated steel sheet
[J].
锌铝镁镀层钢板的研究进展
[J].
Initial corrosion behavior of carbon steel and weathering steel in Nansha Marine atmosphere
[J].Along with the increasing pace of marine resource development and strategic deployment of China, the infrastructure materials and deployed aircraft were facing severe salt fog corrosion during the construction process of the South China Sea. Materials damage in this environment is much more serious than that in other marine atmospheric environment. Owing to its location near the equator and the direct impact of solar radiation, Nansha marine atmosphere is a representative and typical climate with high temperature, high humidity, high salinity and high radiation. However, there has been lack of material corrosion data and relevant fundamental research until now. Carbon steel is usually one of the most widely used infrastructure materials and reference materials, and its corrosion data exposed to Nansha Islands marine atmosphere is much more important. These corrosion data can not only provide important basis for environmental corrosivity category, but also provide reference for indoor accelerated corrosion test. Therefore, in order to obtain useful information on selected construction materials, adopting the appropriate corrosion protection methods, and predicting the life of metallic structures under service, the exposure test was conducted on carbon steel Q235 and weathering steel Q450NQR1 in Nansha Islands for 2 and 5 months. Thickness loss analysis, macroscopic observation, SEM, XRD, optical profiler and tensile tests were conducted to study the initial corrosion behavior on both sides of Q235 and Q450NQR1 in Nansha marine atmosphere. The results showed that the initial corrosion behavior of both steels at this site was more serious than those at most areas, such as Wanning and Xisha Islands, and the corrosion of skyward of both steels was more serious than that of field-ward. The rust layer formed on field-ward was easier to fall off. After exposure for 2 months, the thickness loss of Q235 was the same as that of Q450NQR1, and corrosion products on both sides were mainly composed of γ-FeOOH, α-FeOOH and Fe3O4; while after 5 months' exposure, the thickness loss of Q235 was much larger than that of Q450NQR1, and corrosion products were mainly composed of γ-FeOOH, α-FeOOH, Fe3O4 and β-FeOOH. The relative composition of β-FeOOH and γ-FeOOH was fewer on the field-ward, and the relative composition of Fe3O4 was fewer on the skyward.
碳钢和耐候钢在南沙海洋大气环境中的初期腐蚀行为
[J].采用腐蚀失重法、宏观形貌观察法、SEM、XRD、白光干涉及拉伸实验等分析手段对碳钢Q235和耐候钢Q450NQR1在南沙大气环境下的初期腐蚀行为进行了研究。结果表明,Q235和Q450NQR1在南沙大气环境中的初期腐蚀比万宁及西沙等海洋大气环境中的腐蚀严重,2种钢的朝天面都比朝地面腐蚀严重,朝地面的锈层更容易脱落。暴晒2个月时,Q235和Q450NQR1的腐蚀失厚相近。暴晒5个月时,Q235的腐蚀失厚明显高于Q450NQR1的腐蚀失厚。2种钢在暴晒2个月时,朝天面和朝地面的腐蚀产物都主要为γ-FeOOH、α-FeOOH和Fe<sub>3</sub>O<sub>4</sub>;而暴晒5个月时,朝天面产物中出现了β-FeOOH,而朝地面β-FeOOH极少。朝天面的产物中Fe<sub>3</sub>O<sub>4</sub>相对含量少于朝地面,γ-FeOOH的相对含量多于朝地面。
Corrosion behavior of Q235 and Q450NQR1 exposed to marine atmospheric environment in Nansha, China for 34 months
[J].Many countries have begun to focus on the development and utilization of marine resources, involving ports, docks, oil production platforms, cross-sea bridges, large ships, and other marine engineering facilities, since beginning of the 21st century. Marine atmospheric corrosion issues encountered during construction put the safety of these marine engineering facilities in jeopardy. The Nansha Islands, which are located in the southernmost part of the South China Sea, are in a typical tropical marine atmosphere environment with no long-term corrosion data. The steel used in marine engineering is the premise of expanding marine space and exploiting marine resources, as well as the guarantee of enhancing marine national defense strength and safeguarding maritime rights and interests. Because of its poor corrosion resistance, the service life has certain limitations. As a result, studying the corrosion mechanism of carbon steel in this typical atmospheric environment after long-term exposure is crucial for engineers. The corrosion behavior of low carbon steel Q235 and weathering steel Q450NQR1 was investigated using the corrosion loss method, macroscopic morphology observation, SEM, XRD, and electrochemical and tensile tests after 34 months of exposure in the Nansha atmospheric environment. The results show that the corrosion dynamic of the two sheets of steel in the marine atmosphere of Nansha Islands can be divided into two stages. The corrosion rate of the second stage is smaller than that of the first stage. Weathering steel Q450NQR1 has demonstrated better corrosion resistance in a short exposure time. The rust layer on the skyward and field-ward sides of mild steel Q235 is thicker than that of weathering steel Q450NQR1 after 21 and 34 months of exposure, and there are more cracks in the rust layer, which could promote oxygen and chloridion diffusion to the substrate and speed up the corrosion process. The main components of corrosion products are γ-FeOOH, α-FeOOH, β-FeOOH, and Fe3O4, the relative contents of each product were different with the extension of exposure time. Furthermore, corrosion on the field-ward sides of the two steel sheets was worse than corrosion on the skyward sides. This is because the rust layer on the field-ward side was easily removed, resulting in a weakened resistance to corrosive medium. With the extension of exposure time, the thickness of the rust layer on the skyward side of carbon steel Q235 and weathering steel Q450NQR1 is increasing, and the tensile strength was gradually reduced. That is, Q235 and Q450NQR1 are more likely to fail owing to the thickening of the rust layer during use resulting in safety accidents.
Q235和Q450NQR1在中国南沙海洋大气环境中暴晒34个月后的腐蚀行为
[J].采用腐蚀失重法、宏观形貌观察法、SEM、XRD、电化学及拉伸实验等分析手段对Q235和Q450NQR1在南沙大气环境中暴晒21和34个月后的腐蚀行为进行研究。结果表明,2种钢在南沙海洋大气环境中腐蚀动力学过程分为2个阶段,第二阶段腐蚀速率较第一阶段小。耐候钢Q450NQR1在短期内就已体现出更好的耐蚀性。暴晒21和34个月后,Q235朝天面和朝地面的锈层均比Q450NQR1的厚,且锈层中的裂纹更多,利于O<sub>2</sub>和Cl<sup>-</sup>向基体扩散,加速腐蚀过程。2种碳钢朝天面和朝地面锈层的主要成分组成为γ-FeOOH、α-FeOOH、β-FeOOH和Fe<sub>3</sub>O<sub>4</sub>,各产物的相对含量随着暴晒时间的延长都有一定的差异。此外,2种钢的朝地面均比朝天面的腐蚀严重,这是由于朝地面的锈层极易脱落,使其对腐蚀介质的阻碍作用减弱。随着暴晒时间的延长,碳钢Q235和耐候钢Q450NQR1表面锈层不断增加,抗拉强度逐渐降低。即在使用过程中随着锈层的增厚,Q235和Q450NQR1钢越容易失效,引发安全事故。
Corrosion resistance of zinc-magnesium coated steel
[J].
In situ Raman study of the corrosion of zinc-coated steel in the presence of chloride: I. Characterization and stability of zinc corrosion products
[J].
Corrosion mechanisms for zinc exposed to the atmosphere
[J].
Calculations of the cathodic current delivery capacity and stability of crevice corrosion under atmospheric environments
[J].
Corrosion behavior of zinc-aluminum-magnesium coated steel in simulated marine atmosphere
[J].