南极洲拥有得天独厚的自然地理位置和环境,具有丰富的油气矿产资源和极高的科学研究价值。极地资源的合理开发与利用是全世界共同面临的重大问题,而极地装备是科学认知极地、合理开发利用极地资源的基础保障[1]。极地大气条件下材料的腐蚀失效威胁着极地装备的安全使用。低合金钢具有较高的强度以及良好的耐腐蚀性能,被广泛应用于海洋工程建设中,其大气腐蚀特性在世界范围内得到了广泛关注,但目前仍缺乏对南极低温环境下低合金钢大气腐蚀行为的认识,南极极端恶劣的大气环境对低合金钢的安全服役提出了严峻挑战。
尽管极地大气环境中金属表面长时间被冰雪覆盖并处于低温环境,但金属仍有不同程度的腐蚀[8~10]。Rosales和Fernández[11]在南极半岛阿根廷的Jubany站研究了钢、Zn、Cu和Al的腐蚀行为,结果表明,在海盐存在的情况下,冰下的金属表面会形成液态水层,由此腐蚀可以在低于0 ℃时发生。同时,含盐颗粒的沉积在南极是一个重要的问题,如Cl-的存在可以提高电解液的电导率,加速金属的腐蚀。Brass[12]通过2种氯化物盐和水的三元相图讨论了冰点的降低对腐蚀速率的影响,发现低温环境中含盐颗粒的富集能够降低凝固点,使得腐蚀反应在-50 ℃以下发生。Fu等[13]研究发现,完全冻结的NaCl溶液仍然是导电的,而且存在3种导电路径。Ohanian等[9]也发现南极环境下金属表面的冰层之下存在电化学反应,并测得低合金钢的腐蚀电位为-250 mVSCE。White等[14]发现冰层中温度在-20 ℃时依然存在电化学腐蚀反应。而Bartoň等[15]则认为在污染性的海洋大气环境中低于-5 ℃时发生腐蚀,这是由于液膜中较高的盐浓度延迟了结冰。以往研究均表明,南极低温环境冰层、雪层覆盖下电化学腐蚀过程依然存在。
本课题组前期已对Q235碳钢在南极大气环境中的腐蚀行为进行了报道[20],但是对低合金钢的南极大气环境中的腐蚀行为仍不清楚。本工作分析表征了Q460和Q690低合金高强钢暴露于南极中山站大气环境中1和12个月后的腐蚀速率、腐蚀形貌和腐蚀产物,探讨了低合金钢在南极低温环境中的腐蚀行为与机理。
1 实验方法
1.1 实验材料
本工作使用Q460和Q690低合金钢用于南极室外暴露实验,其主要化学成分如表1所示。试样经打磨抛光后使用4% (体积分数)硝酸酒精刻蚀,并采用Axio-Lab.A1光学显微镜(OM)和Gemini SEM 300扫描电子显微镜(SEM) 进行组织观察。暴露试样尺寸为150 mm × 75 mm × 2 mm,经打磨抛光、除油清洗并干燥后进行称重及尺寸记录。
表1 低合金钢Q460和Q690的化学成分 (mass fraction / %)
Table 1
| Steel | C | P | Si | Cr | Mn | Ni | V | Nb | Ti | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q460 | 0.09 | 0.01 | 0.36 | 0.023 | 1.42 | - | 0.066 | 0.034 | 0.012 | 0.014 | Bal. |
| Q690 | 0.06 | 0.005 | 0.21 | 0.51 | 1.76 | 0.134 | - | - | - | 0.3-0.4 | Bal. |
1.2 室外暴露环境
试样准备完毕后在南极中山站大气试验场(69°22ʹ24ʺ S, 76°22ʹ40ʺ E)进行现场暴露,如图1所示,样品正面与水平面成45°进行暴晒,时间为2019年1月~2020年1月,共12个月。南极中山站大气试验场的气象数据如下:年平均温度为-9.5 ℃,年平均相对湿度为61%;在南极暖季(10月~2月),日最高温度高于0 ℃;在南极寒季(3~9月),日最低温度可达-36.4 ℃;在2019年1月份下雪的天次只有1 d,按照海水在南极的冻结温度在-1.9 ℃计算,冰雪冻-融循环天次共有7 d[21]。根据King等[22]所提出的标准,在寒冷环境中以温度高于-10 ℃和相对湿度高于50%计算表面润湿时间(TOW),南极暖季达到672 h/月,南极寒季低至24 h/月。因为没有雨水,盐不会被冲走,导致南极地区含盐颗粒的沉积量很高。此外,中山站有极昼和极夜现象,连续白昼时间54 d,连续黑夜时间58 d。
图1
图1
本实验中的南极中山站暴露地点
Fig.1
Exposure site in Zhongshan station in Antarctic continent
1.3 表征方法
试样取回后,首先使用Canon 6D MarkII数码相机对暴露后的低合金钢的宏观腐蚀形貌进行观察,然后刮取锈层用作成分分析。采用500 mL HCl、3.5 g C6H12N4及去离子水配置成1000 mL的除锈液,将带有腐蚀产物的完整试样浸泡在除锈液中,用超声波清洗机超声除去腐蚀产物,除锈后的试样依次使用去离子水、无水乙醇冲洗,吹风机冷风吹干,并称重记录。
腐蚀速率测试:大气腐蚀研究中,室外暴晒环境中金属材料的腐蚀失重速率计算为:
式中,v表示腐蚀速率(mm/a),mt 表示除锈后试样的质量(g),m0表示暴晒前试样的质量(g),S表示试样的表面积(cm2),ρ表示低合金钢的密度(约为7.86 g/cm3),t表示暴露腐蚀实验时间(h)。
腐蚀形貌观察:使用Gemini SEM 300型SEM对除锈前后的试样表面进行观察。截面试样采用环氧树脂封装后用砂纸依次打磨至5000号并抛光,使用去离子水、无水乙醇冲洗,冷风吹干后待用。使用SEM和KEYENCE VK-X250型激光共聚焦显微镜(CLSM)观察除锈后低合金钢的表面腐蚀形貌,并随机选取100个点蚀坑统计深度分布信息。
腐蚀产物分析:刮取暴露12个月后的锈层在研钵中研磨均匀,使用D8 Advance X射线衍射仪(XRD)进行物相分析,并使用Jade软件和RIR (reference intensity ratio)值法对锈层相组成进行定性和定量分析。工作电压设置为50 kV和30 mA,扫描角度为10°~90°,步长为0.03°,扫描速率为3.6°/min。使用Renishaw in Via型Raman光谱仪对腐蚀产物的相组成与分布进行分析,扫描范围100~1700 cm-1。
2 实验结果
2.1 显微组织观察
Q460和Q690低合金钢的微观组织如图2所示。Q460钢主要为晶粒细小的铁素体和珠光体组织,Q690钢主要为板条贝氏体组织。
图2
图2
Q460和Q690低合金钢微观组织的OM和SEM像
Fig.2
OM (a, b) and SEM (c, d) images of microstructures of Q460 (a, c) and Q690 (b, d) low alloy steels
2.2 腐蚀速率测试
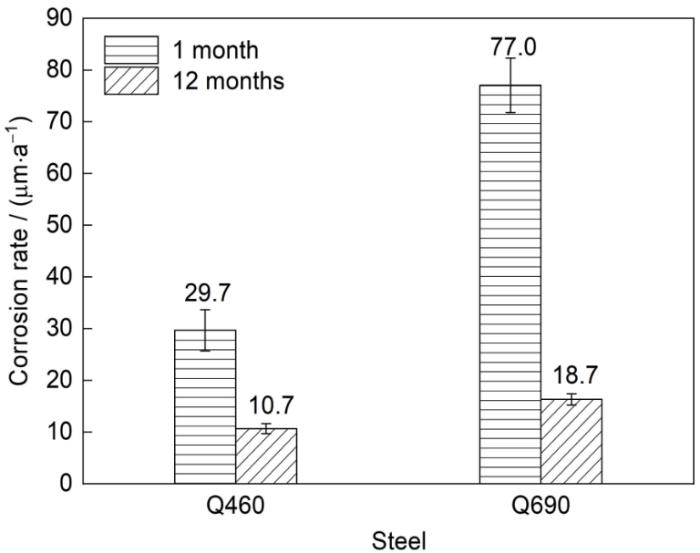
Q460和Q690低合金钢暴露1和12个月后的腐蚀速率变化如图3所示。在南极大气环境下,暴露1个月时,Q460和Q690低合金钢的腐蚀速率分别为29.7和77.0 μm/a,腐蚀速率差异较大。暴露12个月时,Q460和Q690低合金钢的腐蚀速率分别为10.7和18.7 μm/a,腐蚀速率均大幅度降低。表明南极低温环境下冰层、雪层覆盖下电化学腐蚀过程依然可以发生。其中,Q460钢表现出更好的耐腐蚀性能。而在暴露初期,2种低合金钢的腐蚀速率均较高,因为实验用钢暴露1个月时处于南极暖季,由于冰雪的冻-融循环导致液膜的长周期存在从而加速了腐蚀的进程。而在南极寒季,冰层持续覆盖金属表面,冰层下电化学腐蚀过程减缓,南极大气环境的大幅季变导致材料腐蚀速率在不同的暴露周期内有较大的差异。
图3
图3
Q460和Q690低合金钢的腐蚀速率随暴露时间的变化
Fig.3
Variation in the corrosion rates of Q460 and Q690 low alloy steels as a function of exposure time
2.3 腐蚀形貌分析
2.3.1 宏观形貌
Q460和Q690低合金钢暴露于大气后形成的腐蚀产物表面形貌如图4所示。锈层的颜色由腐蚀产物的结构和特征所决定,暴露不同时间后其颜色会发生明显的变化。暴露1个月时,Q460钢正面腐蚀产物覆盖并不完整,且不均匀,试样边缘处和固定试样片材的圆孔周围腐蚀较为严重,腐蚀产物呈橘黄色和棕褐色,背面锈层较少,仍能看到未腐蚀的基体。Q690钢相比于Q460钢腐蚀更为严重,腐蚀产物呈棕褐色,背面腐蚀较为轻微,也呈现出均匀腐蚀的趋势。暴露12个月时,Q460和Q690钢已完全被腐蚀产物所覆盖,正面腐蚀产物呈棕褐色,背面出现黑色块状腐蚀产物,而试样正面腐蚀产物更加均匀致密。
图4
图4
南极大气环境下Q460和Q690低合金钢暴露不同时间的正反面宏观形貌
Fig.4
Macromorphologies of the corrosion products on skyward (a, c, e, g) and groundward (b, d, f, h) surfaces of Q460 (a, b, e, f) and Q690 (c, d, g, h) low alloy steels exposed to Antarctic atmosphere for 1 month (a-d) and 12 months (e-h)
2.3.2 表面腐蚀产物微观形貌
图5为南极大气环境下Q460和Q690低合金钢暴露不同时间的微观形貌。暴露1个月后,Q460和Q690钢表面存在多种形态的腐蚀产物,局部仍可见未腐蚀的钢基体。Q460钢较大倍数下观察到相对平整的块状腐蚀产物,同时产生了较大裂纹。局部腐蚀产物放大后的微观形貌如图5b2和b3所示,可见大量细长且有序排列的棱柱状的β-FeOOH,而且棱柱状微观产物之间有较多缝隙,这有利于侵蚀性离子的进入[23]。Q690钢表面出现了片层状的γ-FeOOH[24],局部有微裂纹出现。通常来说,β-FeOOH和γ-FeOOH作为暴露初期所形成的腐蚀产物还原性较强,能够促进腐蚀的发生[25]。暴露12个月后,Q460和Q690钢表面完全被腐蚀产物所覆盖。如图5c3所示,Q460钢表面出现较多花板状α-FeOOH,且表面裂纹不再明显。Q690钢表面较大倍数下仍显示出大量裂纹(图5d1),表面腐蚀产物相对平整光滑,在裂纹周围腐蚀产物主要为棉球状和羽毛状的γ-FeOOH。
图5
图5
南极大气环境下Q460和Q690低合金钢暴露不同时间的微观形貌
Fig.5
Micromorphologies of corrosion products formed on Q460 (a1-a3, c1-c3) and Q690 (b1-b3, d1-d3) low alloy steels exposed to Antarctic atmosphere for 1 month (a1-a3, b1-b3) and 12 months (c1-c3, d1-d3) with different magnifications
2.3.3 截面微观形貌
图6为Q460和Q690低合金钢暴露于南极大气环境中所形成的腐蚀产物的截面形貌。可以看出,在暴露初期Q460和Q690钢所形成的锈层厚度在50 μm左右,Q460钢锈层中间部分和Q690钢锈层与钢基体间均产生了明显的裂纹。暴露12个月后,Q460钢上所形成的锈层变得致密连续,裂纹较少,厚度约为150 μm,没有出现明显的分层现象。Q690钢上所形成的锈层出现大量孔洞及裂纹,裂纹沿纵向分布,锈层呈现出脱落的趋势。
图6
图6
南极大气环境下Q460和Q690低合金钢暴露不同时间的截面微观形貌
Fig.6
Cross-sectional morphologies of Q460 (a, b) and Q690 (c, d) low alloy steels exposed to Antarctic atmosphere for 1 month (a, c) and 12 months (b, d)
2.3.4 去除腐蚀产物后腐蚀形貌分析
图7为南极大气环境下Q460和Q690低合金钢暴露不同时间的表面腐蚀形貌。在暴露1个月后,Q460钢和Q690钢出现了不同程度的局部腐蚀现象。2者正面均比背面腐蚀严重,点蚀坑有横向扩展的趋势,背面所形成的点蚀坑深度小于正面,而点蚀坑数量多于正面。对于Q460钢,背面所形成的点蚀坑深度最浅,同时数量也较多。暴露12个月时后,Q460和Q690低合金钢表面整体呈现均匀腐蚀,基本已看不到未腐蚀的钢基体。而Q690钢表面仍存在少量点蚀坑(图7d1~d3)。由于暴露初期出现了明显的局部腐蚀,而在长周期暴露后,低合金钢表面发生均匀腐蚀,因此统计了Q460和Q690低合金钢暴露1个月后的表面点蚀坑深度分布。为了消除表面不平整带来的影响,只统计了深度超过5 μm的点蚀坑。如图8所示,Q460钢点蚀坑深度集中于5~15 μm,深坑更少,而Q690钢深度超过20 μm的深坑相对更多,表明Q460钢在南极低温环境中耐点蚀能力更强。此外,2种低合金钢的背面深坑深度均略小于正面,这可能是由于南极低温环境下冰层雪层的冻-融循环为金属表面提供了持续的Cl-。以上结果表明,低合金钢暴露于南极大气环境中在初期出现了明显的点蚀现象,并随暴露时间的延长,点蚀坑横向扩展,彼此相连,点蚀向均匀腐蚀转变。
图7
图7
南极大气环境下Q460和Q690低合金钢暴露不同时间的表面腐蚀形貌
Fig.7
Surface morphologies and 3D topographies of Q460 (a1-a4, c1-c4) and Q690 (b1-b4, d1-d4) low alloy steels exposed to Antarctic atmosphere for 1 month (a1-a4, b1-b4) and 12 months (c1-c4, d1-d4)
(a1-d1, a2-d2) skyward (a3-d3, d4-d4) groundward
图8
图8
南极大气环境下Q460和Q690低合金钢暴露1个月后的表面点蚀坑深度分布统计
Fig.8
Distribution statistics of surface pits depth of Q460 and Q690 low alloy steels exposed to Antarctic atmosphere for 1 month
2.4 锈层成分分析
2.4.1 XRD
图9a和b分别为Q460和Q690低合金钢暴露于南极大气环境12个月后形成的锈层粉末的XRD谱和所对应物相组成占比。由于XRD难以区分Fe3O4和γ-Fe2O3,而且2者可以相互转化,因此以Fe3O4/γ-Fe2O3表示2者的总量。同时计算了α / γ* (α代表α-FeOOH,γ*代表γ-FeOOH、β-FeOOH与Fe3O4/γ-Fe2O3的总量[26]。α / γ*代表锈层保护性指数,α / γ*的值越大,锈层保护性越好),如图9c所示。可以看出,低合金钢在南极大气环境中所形成的腐蚀产物主要为α-FeOOH、γ-FeOOH、β-FeOOH以及Fe3O4/γ-Fe2O3。对于Q460钢,γ-FeOOH和β-FeOOH占据了主要的物相组成,Fe3O4/γ-Fe2O3的含量仅有1%左右。对于Q690钢,正面和背面物相成分差距较大,正面γ-FeOOH是腐蚀产物的主要成分,而且背面Fe3O4/γ-Fe2O3的含量更多。相比于Q460钢,α-FeOOH的含量较少,其α / γ*也低于前者。上述结果表明,低合金钢暴露于南极大气环境中所形成的腐蚀产物除了α-FeOOH、γ-FeOOH和Fe3O4/γ-Fe2O3以外,存在大量β-FeOOH,而且Q460钢的锈层的保护性比Q690钢更好,Q690钢背面所形成的锈层的保护性最差。
图9
图9
Q460和Q690低合金钢暴露于南极大气环境形成的腐蚀产物的物相组成
Fig.9
XRD spectra (a), proportion of each phase (b), and the protective ability (c) of the corrosion products formed on Q460 and Q690 low alloy steels (α represents α-FeOOH; γ* represents total of γ-FeOOH, β-FeOOH, and Fe3O4/γ-Fe2O3)
2.4.2 Raman光谱分析
为了确定Q460和Q690低合金钢暴露于南极大气环境后形成的锈层的物相分布,对锈层截面进行检测,检测位置如图6所示黄色标记点1#~10#,检测结果如图10所示,Raman光谱的峰值位置参见文献[27]。检测结果表明,暴露初期,腐蚀产物物相主要为γ-FeOOH和α-Fe2O3。而α-Fe2O3的存在可能归因于Raman测量的激光功率引起的局部加热导致的Fe3O4/γ-Fe2O3的氧化而成[28],因此暴露初期腐蚀产物物相主要为γ-FeOOH和Fe3O4/γ-Fe2O3。暴露12个月后,随着腐蚀的加深,锈层变厚,不同的相在锈层中有了明显的分布。对于Q460钢,锈层内部如图10a-Point 3#所示,对应的物相为α-FeOOH[29]。锈层中间层如图10a-Point 4#所示,对应的物相为β-FeOOH[30]。锈层外层如图10a-Point 5#所示,对应的物相为γ-FeOOH。对于Q690钢,锈层内部Raman光谱如图10b-Point 8#所示,对应物相为α-FeOOH和Fe3O4/γ-Fe2O3[31]。锈层中间层和外层分别对应β-FeOOH和γ-FeOOH。以上结果表明,暴露初期所形成的γ-FeOOH和Fe3O4/γ-Fe2O3不稳定,随暴露时间的延长,腐蚀产物继续生长并转化,内部形成致密稳定的α-FeOOH,锈层中间层主要为β-FeOOH,而外层主要为γ-FeOOH。对于Q690钢,在锈层与基体界面处存在部分Fe3O4/γ-Fe2O3,同时α-FeOOH的含量少于Q460钢,表明Q460钢暴露于南极大气环境所形成的锈层内层的致密性更大,保护性更好。内外锈层物相的不同可能与合金元素的加入以及腐蚀介质中Cl-的浓度有关[32]。
图10
图10
低合金钢Q460和Q690暴露于南极大气环境形成的腐蚀产物的物相分布
Fig.10
Phase distributions of the corrosion products (see in Fig.6) formed on Q460 (a) and Q690 (b) low alloy steels (A, G, L, and M represent akaganeite (β-FeOOH), goethite (α-FeOOH), lepidocrocite (γ-FeOOH), and megnetite/meghemite (Fe3O4/γ-Fe2O3), respectively)
2.5 腐蚀机理分析
南极低温海洋大气环境季节特征明显,由于降水少,含盐颗粒会沉积在材料表面。在南极暖季,温度和相对湿度较高,冰雪的冻-融循环导致薄液膜长周期存在加速了腐蚀的进程。而在南极寒季,材料表面长周期被冰雪所覆盖,尽管在较低的温度和相对湿度下,材料表面仍然可以形成薄液膜,腐蚀反应仍然可以进行。
随着腐蚀的进行,锈层进一步形成并生长。γ-FeOOH将发生相转变进而转化形成
南极含盐颗粒沉积于材料表面,使得大量Cl-持续进入到薄液膜中,Cl-在锈层/金属界面处的富集一方面导致冰点的降低,另一方面使得局部酸化进而导致β-Fe2(OH)3Cl的形成,再经过不稳定的中间产物绿锈I (GRI)的转化,最终形成β-FeOOH[37]。完整的形成过程如式(
一定含量的Mn存在时,锈层具有阳离子选择性,Mn2+会占据Fe3O4反尖晶石结构的一个八面体中心[39]。通常Mn2+可以和PO
3 结论
(1) Q460和Q690低合金钢在南极大气环境下暴露1个月时腐蚀速率分别为29.7和77.0 μm/a,暴露12个月时,2者的腐蚀速率分别降低至约10.7和18.7 μm/a。
(2) Q460和Q690低合金钢大气暴露12个月后形成的腐蚀产物主要为α-FeOOH、γ-FeOOH、β-FeOOH以及Fe3O4/γ-Fe2O3,锈层内层主要为α-FeOOH和β-FeOOH,锈层外层主要为γ-FeOOH。Q460钢的锈层保护性比Q690钢更好。在南极低温环境下,由于Cl-的存在,2种钢的锈层均产生了较多的β-FeOOH以及裂纹。
(3) 暴露1个月时,Q460和Q690低合金钢均发生了严重的局部腐蚀,2者正面均比背面腐蚀严重,Q690钢比Q460钢腐蚀严重。随暴露时间的延长,2者的腐蚀行为由局部腐蚀向均匀腐蚀转变。
(4) 南极低温环境下,暴露初期处于南极暖季,冰雪冻-融过程导致液膜长周期存在促进了腐蚀的进行且加速局部腐蚀。长周期暴露时,金属表面被冰雪所覆盖,冰层以及锈层对溶解氧及侵蚀性离子阻挡使得腐蚀的发生受到了抑制。
参考文献
Development strategy for polar equipment in China
[J].
我国极地装备技术发展战略研究
[J].当前极地地区的战略地位日益凸显,极地资源开发与利用成为国际社会关注的焦点,而极地装备是科学认知极地、合 理开发利用极地资源的基础保障。极地装备主要包括极地科学装备、极地船舶装备、极地资源开发装备等三大类。针对此三 类装备,本文梳理了国外极地装备技术的发展态势,分析了我国极地装备的发展现状及存在的差距与问题,研判了极地装备 的发展趋势与关键技术,总结了我国极地装备技术领域的发展战略。针对我国极地科学装备不足且冰下观测能力有限、极地 船舶能力建设缺乏以及极地资源开发装备研发设计能力欠缺等问题,从建设北极环境观测与通信导航装备、攻克极地航行船 舶关键技术、发展极地资源勘探关键装备 3 个方面提出了我国面向 2035 年的极地装备重点发展方向及对策建议,以期为 21 世纪下半叶极地夏季商业航线的大规模开发提供装备和技术研究支持。
Antarctic observations: On metal corrosion at three historic huts on Ross Island
[A].
Fundamental understanding on the effect of Cr on corrosion resistance of weathering steel in simulated tropical marine atmosphere
[J].
Optimizing the nickel content in weathering steels to enhance their corrosion resistance in acidic atmospheres
[J].
Weathering steels: From empirical development to scientific design. A review
[J].
Atmospheric corrosion resistance of MnCuP weathering steel in simulated environments
[J].
Progress in research on rust layer of weathering steel
[J].
耐候钢锈层研究进展
[J].综述了近年来关于耐候钢锈层的组成、形成机理及影响因素的研究进展;介绍了耐候钢锈层的保护机理.同时,对耐候钢锈层的研究方向作了展望.
Atmospheric corrosion of reference metals in Antarctic sites
[J].
A Mössbauer and electrochemical characterization of the corrosion products formed from marine and marine-Antartic environments
[J].
Corrosion reaction of iron exposed to the open atmosphere in the antarctic
[J].
Parameters controlling steel and copper corrosion nucleation and propagation in Antarctica
[A].
Freezing point depression by common salts: Implications for corrosion in cold climates
[A].
Corrosion damage in frozen 3.5 wt.% NaCl solution
[J].
Preliminary observations on corrosion of carbon steel in permafrost
[J].
Die kinetik des rostens von eisen in der atmosphäre
[J].
The atmospheric corrosion kinetics of low carbon steel in a tropical marine environment
[J].
Initial corrosion behavior of carbon steel and weathering steel in Nansha marine atmosphere
[J].Along with the increasing pace of marine resource development and strategic deployment of China, the infrastructure materials and deployed aircraft were facing severe salt fog corrosion during the construction process of the South China Sea. Materials damage in this environment is much more serious than that in other marine atmospheric environment. Owing to its location near the equator and the direct impact of solar radiation, Nansha marine atmosphere is a representative and typical climate with high temperature, high humidity, high salinity and high radiation. However, there has been lack of material corrosion data and relevant fundamental research until now. Carbon steel is usually one of the most widely used infrastructure materials and reference materials, and its corrosion data exposed to Nansha Islands marine atmosphere is much more important. These corrosion data can not only provide important basis for environmental corrosivity category, but also provide reference for indoor accelerated corrosion test. Therefore, in order to obtain useful information on selected construction materials, adopting the appropriate corrosion protection methods, and predicting the life of metallic structures under service, the exposure test was conducted on carbon steel Q235 and weathering steel Q450NQR1 in Nansha Islands for 2 and 5 months. Thickness loss analysis, macroscopic observation, SEM, XRD, optical profiler and tensile tests were conducted to study the initial corrosion behavior on both sides of Q235 and Q450NQR1 in Nansha marine atmosphere. The results showed that the initial corrosion behavior of both steels at this site was more serious than those at most areas, such as Wanning and Xisha Islands, and the corrosion of skyward of both steels was more serious than that of field-ward. The rust layer formed on field-ward was easier to fall off. After exposure for 2 months, the thickness loss of Q235 was the same as that of Q450NQR1, and corrosion products on both sides were mainly composed of γ-FeOOH, α-FeOOH and Fe3O4; while after 5 months' exposure, the thickness loss of Q235 was much larger than that of Q450NQR1, and corrosion products were mainly composed of γ-FeOOH, α-FeOOH, Fe3O4 and β-FeOOH. The relative composition of β-FeOOH and γ-FeOOH was fewer on the field-ward, and the relative composition of Fe3O4 was fewer on the skyward.
碳钢和耐候钢在南沙海洋大气环境中的初期腐蚀行为
[J].采用腐蚀失重法、宏观形貌观察法、SEM、XRD、白光干涉及拉伸实验等分析手段对碳钢Q235和耐候钢Q450NQR1在南沙大气环境下的初期腐蚀行为进行了研究。结果表明,Q235和Q450NQR1在南沙大气环境中的初期腐蚀比万宁及西沙等海洋大气环境中的腐蚀严重,2种钢的朝天面都比朝地面腐蚀严重,朝地面的锈层更容易脱落。暴晒2个月时,Q235和Q450NQR1的腐蚀失厚相近。暴晒5个月时,Q235的腐蚀失厚明显高于Q450NQR1的腐蚀失厚。2种钢在暴晒2个月时,朝天面和朝地面的腐蚀产物都主要为γ-FeOOH、α-FeOOH和Fe<sub>3</sub>O<sub>4</sub>;而暴晒5个月时,朝天面产物中出现了β-FeOOH,而朝地面β-FeOOH极少。朝天面的产物中Fe<sub>3</sub>O<sub>4</sub>相对含量少于朝地面,γ-FeOOH的相对含量多于朝地面。
The initial corrosion behavior of carbon steel exposed to the coastal-industrial atmosphere in hongyanhe
[J].The atmospheric corrosion of carbon steel is an extensive topic that has been studied by many authors who have proposed many mechanisms and techniques for studying the phenomena involved and have reported long term exposure data in many different regions throughout the world. However, there are few literatures that have discussed the corrosion results of carbon steel exposed for short-term time which can contribute to the understanding of the initial corrosion mechanisms. Therefore in this work, mass-loss measurement, SEM, XRD, infrared spectroscopy and electrochemical techniques have been used to investigate the initial corrosion evaluation of carbon steel exposed to a coastal-industrial atmospheric environment in Hongyanhe. Mass-loss results show that the short-term corrosion kinetic of carbon steel is in good fitting with linear function, and the average corrosion rate fluctuates over time and don't show the downward trend observed in long-term exposure experiments. Lepidocrocite, goethite and magnetite are identified in corrosion products formed on the surface of exposed carbon steel samples. The content of lepidocrocite shows a decreasing trend over exposure time, while goethite is the opposite. Magnetite appears in the later stages and keeps stable in amount. Pitting and an irregular localized corrosion can be observed clearly on the surface of carbon steel specimens exposed for 10 d. The corrosion product at pitting regions is circular flowery shape which varies in details as the physical and chemical environments change. The rust layer grows over time and eventually covers the entire surface of carbon steel samples exposed for more than 60 d, yet its thickness is uneven. The surface of rust layer has many nest-shaped structures that can't barricade the physical transmission effectively. The protective effect of rust layer has been further discussed in combination with electrochemical results.
碳钢在红沿河海洋工业大气环境中的初期腐蚀行为
[J].利用腐蚀失重、SEM、XRD、红外光谱、电化学方法对碳钢在红沿河海洋工业大气环境中的初期腐蚀行为进行研究。结果表明,碳钢在该环境中的初期腐蚀动力学符合线性规律,腐蚀速率呈现波动变化。腐蚀产物的成分在早期阶段主要为γ-FeOOH和α-FeOOH,随后出现了一定含量的Fe<sub>3</sub>O<sub>4</sub>,γ-FeOOH的含量随着时间呈现减小趋势,而α-FeOOH的变化相反。碳钢腐蚀10 d后的表面主要为点蚀和不规则局部腐蚀形貌,点蚀区的形貌因具体微环境的差异而不同。腐蚀60 d后的材料表面基本覆盖了腐蚀产物,但是锈层厚度不均匀,而且表面有很多巢结构,这种结构不仅容易聚集污染物而且有利于传质过程的进行,减弱了锈层的保护作用。结合电化学测试结果,进一步讨论了腐蚀产物层的保护作用。
Effect of ion selective property on protective ability of rust layer formed on weathering steel exposed in the marine atmosphere
[J].
锈层离子选择性对耐候钢抗海洋性大气腐蚀性能的影响
[J].为阐明耐候钢表面稳定锈层的抗大气腐蚀机制,研究了人工制备锈蚀层的离子选择性.实验结果表明,在海洋性大气中腐蚀4 a的耐候钢表面的锈层产生了分层现象.其内锈层具有较好的阳离子选择性能,阻碍了Cl-的进入;合金元素在钢腐蚀过程中的重新分配提高了锈层和近界面基体中合金元素的含量,保证了该耐候钢在近海大气中具有较好的抗大气腐蚀性能.
Corrosion mechanism of materials in three typical harsh marine atmospheric environments
[J].
几种苛刻海洋大气环境下的海工材料腐蚀机制
[J].以南极低温高辐照冰雪凝-融环境、南海高温高湿高盐雾环境以及滨海氯-霾耦合环境3种典型环境为研究对象,开展了典型海工材料的腐蚀行为研究。结果表明,南极低温环境下冰层、雪层覆盖下电化学腐蚀过程依然可以发生,冰雪凝-融过程导致液膜长周期存在促进了腐蚀的进行且加速局部腐蚀。南海高温高湿高盐雾环境下有色金属材料表面存在化学氧化和电化学腐蚀协同作用机制,不同铝合金的局部腐蚀萌生扩展驱动力不同 (即扩散与电荷转移、氢致沿晶裂纹、腐蚀产物楔入效应),表面润湿时间和Cl<sup>-</sup>协同作用导致腐蚀动力学偏离幂函数规律。滨海氯-霾耦合环境下NH<sub>4</sub><sup>+</sup>加速腐蚀的关键控制因素为缓冲效应导致的持续供H<sup>+</sup>,Cl<sup>-</sup>、NH<sub>4</sub><sup>+</sup>、NO<sub>3</sub><sup>-</sup>协同作用下镁合金发生“类自催化点蚀”。
Review of antarctic landfast sea ice observations
[J].
Studies in Antarctica help to better define the temperature criterion for atmospheric corrosion
[A].
Corrosion mechanisms of mild steel in chloride-rich atmospheres
[J].
An attempt to classify the morphologies presented by different rust phases formed during the exposure of carbon steel to marine atmospheres
[J].
Electrochemical behavior of rust formed on carbon steel in a wet/dry environment containing chloride ions
[J].
Corrosion evolution and stress corrosion cracking behavior of a low carbon bainite steel in the marine environments: Effect of the marine zones
[J].
SEM/micro-Raman characterization of the morphologies of marine atmospheric corrosion products formed on mild steel
[J].
Raman mapping of corrosion products formed onto spring steels during salt spray experiments. A correlation between the scale composition and the corrosion resistance
[J].
Raman spectra of possible corrosion products of iron
[J].
Environmental conditions for akaganeite formation in marine atmosphere mild steel corrosion products and its characterization
[J].
A study on the initial corrosion behavior of carbon steel exposed to a simulated coastal-industrial atmosphere
[J].Carbon steels as common structural material have been widely used for basic facilities with the development of the city. In these service environments, carbon steel would inevitably encounter the atmospheric corrosion. Especially, the corrosion of carbon steels exposed to coastal-industrial atmosphere is very outstanding. However, the initial corrosion mechanism of carbon steel subjected to coastal-industrial environment still need to be clarified, which would be vital for predicating the subsequent corrosion process. In addition, although many scholars studied the synergism of SO2 and Cl-, which obviously accelerates the corrosion of steel and reduces its service life, there is few research about the effect of the synergism of SO2 and Cl- (in different proportion) on the early corrosion behavior of the carbon steel. Therefore, it is of great importance to investigate the initial corrosion mechanism of carbon steel and the effect of the synergism of SO2 and Cl- (in different proportion) in the coastal-industrial atmosphere. In present work, the initial corrosion behavior of Q235 carbon steel exposed to a simulated coastal-industrial atmosphere has been studied by weight loss, XRD, SEM and electrochemical measurements. Also, the effect of the synergism of SO2 and Cl- (in different proportion) on the early corrosion behavior of Q235 car bon steel has been investigated. The results indicate that the initial corrosion behavior of carbon steel exposed to a simulated coastal-industrial atmosphere presented a transition from corrosion acceleration to deceleration, and the kinetics of accelerated corrosion process followed the empirical equation D=Atn. A double-layered corrosion product was formed on the surface of carbon steel after 24 h: the loose outer layer and relative dense inner layer; the synergistic effect between SO2 and Cl- accelerated the corrosion of carbon steel. However, the change in the ratio of SO2 and Cl- had no significant effect on the corrosion loss of carbon steel, and had not changed the composition of corrosion products formed on carbon steel surface. SO2 caused the corrosion morphology of carbon steel to tend to uniform corrosion.
碳钢在模拟海洋工业大气环境中初期腐蚀行为研究
[J].采用失重分析、X射线衍射分析、扫描电镜分析及电化学测试分析方法对Q235碳钢在模拟海洋工业大气环境中的初期腐蚀历程和机理开展深入研究,并着重探究了不同比例SO<sub>2</sub>和Cl<sup>-</sup>的协同效应对碳钢初期腐蚀行为机制的影响。结果表明,Q235碳钢在模拟海洋工业大气环境中的初期腐蚀呈现由加速过程向减速过程转化的特点,且加速过程的腐蚀动力学仍遵循幂函数规律D=At<sup>n</sup>;腐蚀24 h后,腐蚀产物呈现双层结构,即疏松的外层和相对致密的内层。SO<sub>2</sub>和Cl<sup>-</sup>的协同效应会加速碳钢的腐蚀,但二者比例的变化对碳钢腐蚀失重影响并不明显,也没有改变腐蚀产物成分,SO<sub>2</sub>促使碳钢腐蚀形态趋向于均匀腐蚀。
The mechanism of atmospheric rusting and the protective amorphous rust on low alloy steel
[J].
Über die Wirkung des Magnetits beim atmosphärischen Rosten und beim Unterrosten von Anstrichen
[J].
Formation of magnetite rust particles by reacting iron powder with artificial α-, β- and γ-FeOOH in aqueous media
[J].
Formation of magnetite in the presence of ferric oxyhydroxides
[J].
Mechanisms of formation and structure of green rust one in aqueous corrosion of iron in the presence of chloride ions
[J].
Effect of small compositional changes on marine immersion corrosion of low alloy steels
[J].
Study on the rusting evolution and the performance of resisting to atmospheric corrosion for Mn-Cu steel
[J].The rust evolutions of the corrosion mass gain, the corrosion rate, the chemical composition, and the cross-section of the rust layer versus cycles of dry/wet alternate corrosion test were summarized on the basis of the study progress on the rust layer of Mn-Cu weathering steel. The validity of the cyclic dry/wet corrosion acceleration test simulating the atmospheric corrosion has also been discussed. Moreover, we have described the principle of synergistically improving the corrosion resistance of the rust layer resisting to both coastal atmospheric corrosion and industrial acidic rain atmospheric corrosion through adding alloying elements Cu, Mn and P. Meanwhile, the mechanism of P in the rust layer and the relationship of the ion-selectivity of Mn-Cu steel rust layer and its structure have been discussed.
Mn-Cu钢大气腐蚀锈层演化规律及其耐候性的研究
[J].
Rust layer formed on low carbon weathering steels with different Mn, Ni contents in environment containing chloride ions
[J].