有研究表明,采用等离子沉积[12]、水热处理[13]等表面改性方法在Ni-Ti合金表面构建功能性涂层,可以促进支架表面的内皮化,以抑制发生支架内再狭窄,同时还能够提高Ni-Ti合金的耐腐蚀性能,减少Ni2+的释放。例如,Yang等[12]采用等离子沉积技术在Ni-Ti合金表面沉积TiN涂层,改善了内皮细胞功能,对Ni2+释放有一定阻止作用。Zhao等[13]通过水热处理法在Ni-Ti合金表面构建TiO2与Ti4Ni2O组成的纳米纺锤体,增强了内皮细胞的迁移能力,在促进内皮细胞功能表达的同时,减少了Ni2+的释放。然而,这些涂层往往存在与合金基体材料的结合力差,因而在支架使用过程中易发生剥落等问题[14,15]。因此,发展自身具有抑制支架内再狭窄功能并进一步提高耐蚀性能的新型Ni-Ti合金具有重要的临床应用价值。
本工作基于合金化策略,在医用Ni-Ti合金成分的基础上制备了Ni-Ti-Cu合金,并以商用Ni-Ti合金作为对照组,采用电化学实验分析添加Cu对Ni-Ti合金在模拟人体血液中腐蚀行为的影响。通过细胞增殖实验、划痕实验、Transwell实验及小管生成实验,系统研究了Ni-Ti-Cu合金对人脐静脉内皮细胞(HUVECs)分化的促进作用。此外,还采用动态凝血实验,进一步评估了Ni-Ti-Cu合金的抗凝血性能。旨在全面展示Ni-Ti-Cu合金的抑制支架内再狭窄功能及耐蚀性能,为新型含铜Ni-Ti合金血管支架材料的开发提供科学依据。
1 实验方法
1.1 材料制备
采用真空感应炉分别制备Ni-Ti和Ni-Ti-Cu合金,合金的化学成分分别为:50.48Ni-49.52Ti和42.79Ni-50.92Ti-6.29Cu (原子分数,%)。合金在真空熔炼炉中反复熔炼6次而成,以保证合金成分的均匀性。熔炼后的合金锭在1000℃均匀化退火12 h,并在850℃进行热锻,再冷拔为直径7 mm的圆棒,最后切割成1 mm厚的圆片。所有样品在实验前均用SiC砂纸逐级打磨至2000#,用去离子水冲洗后,再用酒精清洗,干燥后得到实验用样品。
1.2 显微组织与相组成表征
通过SSX-550扫描电子显微镜(SEM)观察Ni-Ti与Ni-Ti-Cu合金的微观形貌,工作电压为20 kV。采用D/max 2500 X射线衍射仪(XRD)分析Ni-Ti与Ni-Ti-Cu合金显微组织中的相组成,辐射源为Cu靶Kα 射线,工作电压为40 kV,电流为40 mA,扫描速率为10°/min。用Jade 6.0软件对XRD数据进行相组成分析。
1.3 表面自由能测试
采用JC-2000C型静滴接触角测量仪对样品的表面自由能进行测试。利用微量进样器将去离子水、甘油(C3H8O3)、溴代萘(C10H7Br)缓缓滴于样品表面,当液滴与样品表面接触后,立即采集图像并计算样品的接触角(θ),基于OWRK/Fowkes方法计算样品的表面自由能。表面自由能按照Young-Dupre方程进行计算[31]:
表面自由能由极性分量与色散分量组成:
式中,γsv、γlv分别为固-气界面与液-气界面的表面自由能,
1.4 电化学实验
电化学实验在Reference 600TM上完成,电解质溶液采用模拟人体血液[32]。首先进行开路电位(OCP)测试,之后进行电化学阻抗谱(EIS)测试,从105 Hz下降到10-2 Hz。EIS测试结束后,以0.5 mV/s的扫描速率进行动电位测量,范围为-0.5~2.0 V。使用Gamry Echem Analyst软件分析测试结果,以获得腐蚀电位(Ecorr)、腐蚀电流密度(icorr)和点蚀电位(Epit)。利用ZsimDemo软件对EIS进行电路拟合,以获得电路图中元器件参数。所有测试至少重复3次,以确保结果的可重复性。
1.5 体外生物学实验
1.5.1 细胞培养
使用HUVECs作为模型细胞,在含抗生素(100 U/mL青霉素、100 μg/mL链霉素)和10%胎牛血清的RPMI.1640培养基中于37℃、5%CO2 (体积分数)饱和湿度条件下进行培养。当细胞生长至趋近融合时,使用胰蛋白酶消化,收集细胞并调整细胞浓度至2 × 104 cell/mL,于无菌条件下在样品表面接种细胞进行培养。
1.5.2 浸提液制备
样品经121℃高温高压蒸汽灭菌30 min后,于无菌条件下制备浸提液。以1.25 cm2/mL的浸提比例将样品浸泡于RPMI.1640培养基中,在37℃浸泡72 h后,取出准备好的浸提液即刻进行实验。
1.5.3 细胞增殖实验
将Ni-Ti合金与Ni-Ti-Cu合金样品分别置于48孔板中,设置4个平行样。每孔加入1 × 104个细胞,在37℃、5%CO2条件下分别培养1和3 d。培养结束后,弃去培养板内培养基,加入300 μL含10% CCK-8试剂的培养基,再在37℃、5%CO2条件下培养2 h。取出样品,在Multidkan GO酶标仪上测量450 nm波长处各孔的吸光度(optical density,OD)。
1.5.4 划痕实验
在孔板背面绘制平行线,保证每孔有3条直线穿过。每孔加入5 × 104个细胞,在37℃、5%CO2条件下培养至铺满孔底90%面积。用200 μL枪头垂直于上述平行线轻轻绘制垂线,随后经磷酸盐缓冲溶液(PBS)缓慢冲洗后,加入1 mL无血清的浸提液。在加入浸提液0和24 h后,对同一位置进行拍摄。使用Photoshop软件计算前后2个时间点照片中的划痕区域面积,计算相对迁移面积比率。
1.5.5 Transwell实验
利用Transwell实验可以研究不同材料浸提液对内皮细胞个体迁移能力的影响。实验前,内皮细胞于无血清培养液中饥饿培养24 h。向Transwell上室每孔加入2 × 104个细胞,下室加入600 μL含20%血清的样品浸提液,于37℃、5%CO2下培养24 h。培养结束后,取出Transwell小室,弃去孔中的培养液,用棉签轻轻擦掉上层迁移的细胞,经PBS清洗2次后,在4%多聚甲醛固定30 min。将小室适当风干后,使用0.1%结晶紫染色20 min,经PBS清洗3次后,在FV10-ASW激光共聚焦荧光显微镜下随机挑选5个视野进行拍照并记数。
1.5.6 小管生成实验
将Matrigel基质胶置于4℃溶解,向96孔板中每孔加入50 μL基质胶,于37℃细胞培养箱中孵育30 min进行固化。将内皮细胞消化后,每孔加入100 μL浓度为5 × 105 cell/mL的细胞悬液,并加入100 μL含10%血清的浸提液,于37℃、5%CO2下培养3 h生成小管。在显微镜下随机选取5个视野进行观察拍摄。使用Wimasis Wim Tube软件定量分析小管长度和节点数。
1.5.7 动态凝血实验
采用动态凝血实验评价样品的抗凝血性能。将100 μL抗凝人血滴加到实验样品表面,加入10 μL浓度为0.2 mol/L的CaCl2溶液,启动凝血。分别计时10、30、50、70、90 min后,用2 mL去离子水缓慢流注于样品表面,并轻轻混匀。随后,吸取100 μL的流注液于96孔板中,使用酶标仪在545 nm波长处测量流注液的OD,并绘制动态凝血时间曲线,分析样品表面的血液凝固情况。
1.6 统计学分析
实验数据采用平均值和标准差来表示,每种材料测试3个以上的样品。采用SPSS 13.0软件和GraphPad Prism软件对数据进行统计学分析以得到方差p,p < 0.05时表示数据具有显著性差异,p < 0.01时表明数据差异性极显著。
2 实验结果与讨论
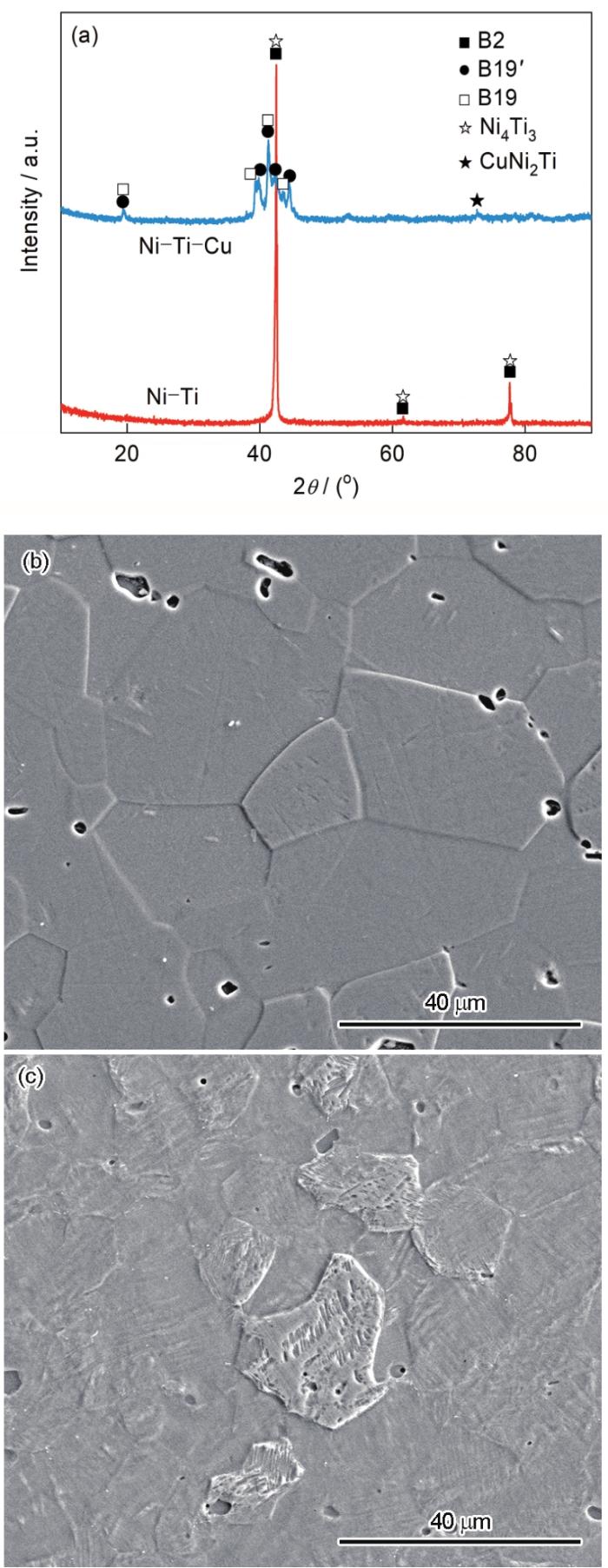
图1
图1
Ni-Ti和Ni-Ti-Cu合金的XRD谱与SEM像
Fig.1
XRD spectra (a) and SEM images of Ni-Ti (b) and Ni-Ti-Cu (c) alloys
植入材料的表面润湿性及其他表面特征(如表面形貌、表面电荷、表面官能团等),对材料与人体组织之间界面上发生的一系列生物学反应具有重要影响,包括蛋白质吸附、与软组织的相互作用以及细菌生物膜的形成[33]。因此,测试了Ni-Ti和Ni-Ti-Cu合金与不同液体的接触角,并基于OWRK/Fowkes方法计算出表面自由能,结果如表1所示。可见,Ni-Ti和Ni-Ti-Cu合金与3种液体的接触角均小于90°,都是亲水性表面。Ni-Ti-Cu合金具有更低的极性力分量与色散力分量,因此具有更低的总表面自由能。有研究[34]表明,具有超亲水表面与超疏水表面的材料都具有良好的血液相容性。Ni-Ti与Ni-Ti-Cu合金均为一般的亲水性表面,因此应该具有相近的血液相容性,但具有较低表面自由能的材料更易于获得优异的抗凝血性能[35]。
表1 不同样品与去离子水、甘油和溴代萘的接触角及表面自由能测量结果
Table 1
| Alloy | θ1 / (°) | θ2 / (°) | θ3 / (°) | ||
|---|---|---|---|---|---|
| Ni-Ti | 53.44 ± 1.94 | 55.53 ± 3.27 | 18.59 ± 0.37 | 11.94 ± 1.31 | 39.31 ± 0.83 |
| Ni-Ti-Cu | 58.36 ± 3.60 | 65.73 ± 3.58 | 19.12 ± 0.88 | 8.99 ± 1.12 | 37.56 ± 1.08 |
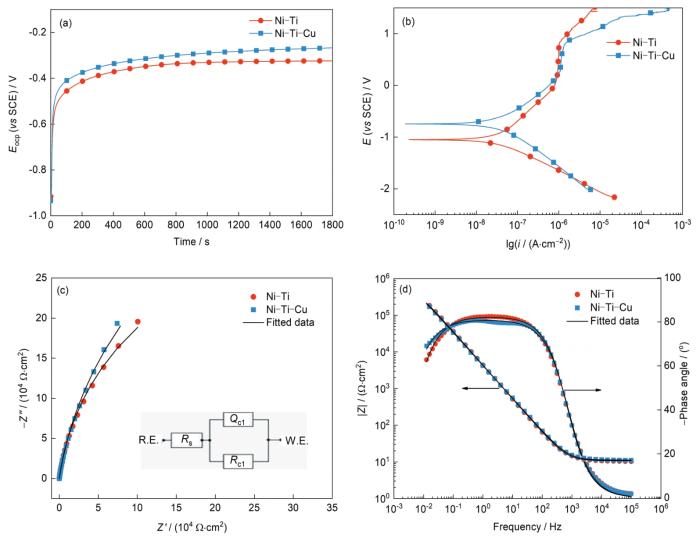
通过电化学实验探究Ni-Ti-Cu合金在模拟人体血液中的耐蚀性能。图2a中的OCP测试结果表明,Ni-Ti-Cu合金较Ni-Ti合金具有更高的开路电位(Eocp),表明Ni-Ti-Cu合金在热力学上发生腐蚀的倾向较低。图2b的动电位极化曲线结果表明,Ni-Ti与Ni-Ti-Cu合金均发生了点蚀,且均表现出活化-钝化-过钝化的特征。Ecorr、icorr和Epit的测试结果如表2所示。可见,Ni-Ti-Cu合金的Ecorr与Epit均高于Ni-Ti合金,icorr低于Ni-Ti合金,表明在动力学上,Ni-Ti-Cu合金耐局部腐蚀与均匀腐蚀的能力均优于Ni-Ti合金,这可能是由于Ni-Ti-Cu合金表面上形成了更为致密的钝化膜[36]。随后,对2种合金进行EIS测试,结果如图2c和d所示。对EIS结果进行拟合,拟合电路图和参数示于图2c插图和表2中,拟合电路图中的Rs、Qc1和Rc1分别表示溶液电阻、常相位角元件和钝化膜电阻。结果表明,Ni-Ti与Ni-Ti-Cu合金的Nyquist图(图2c)在整个测试频率范围内(105~10-2 Hz)均表现为单一的容抗弧,表明2者均为具有单个时间常数的典型电容行为。这可以通过Bode图(图2d)验证,其中2种合金的相位角在宽频率范围内(102~10-1 Hz)均接近90°。此外,Ni-Ti-Cu合金的阻抗弧半径大于Ni-Ti合金,2种合金的Rs与Qc1相近,但Ni-Ti-Cu合金的Rc1较Ni-Ti合金高出1个数量级,表明Ni-Ti-Cu合金的钝化膜具有更加优异的保护能力。以上电化学实验结果表明,Ni-Ti-Cu合金在模拟人体血液中的耐蚀性能要优于Ni-Ti合金。由此可见,Cu的添加提高了Ni-Ti-Cu合金的耐蚀能力。
图2
图2
Ni-Ti和Ni-Ti-Cu合金的电化学测试结果
Fig.2
Electrochemical test results of Ni-Ti and Ni-Ti-Cu alloys
(a) open circuit potential curves (Eocp—open circuit potential)
(b) potentiodynamic polarization curves (i—current density, E—potential)
(c) Nyquist diagram and fitting circuit diagram (Inset shows the equivalent circuit diagram. Z'—real impedance, Z''—imaginary impedance, R.E.—reference electrode, W.E.—working electrode, Rs—solution resistance, Rc1—passive film resistance, Qc1—constant-phase element)
(d) Bode diagram (|Z|—impedance modulus)
表2 从动电位极化曲线与EIS图中获得的电化学参数
Table 2
| Alloy | Ecorr / mV | icorr / (nA·cm-2) | Epit / mV | Rs / (Ω·cm2) | Rc1 / (104 Ω·cm2) | Qc1 / (μΩ-1·s n ·cm-2) |
|---|---|---|---|---|---|---|
| Ni-Ti | -399 ± 62 | 99.7 ± 13.4 | 828 ± 30 | 13.73 ± 1.64 | 6.41 ± 1.20 | 52.3 ± 1.5 |
| Ni-Ti-Cu | -283 ± 52 | 59.5 ± 16.3 | 862 ± 25 | 11.79 ± 1.22 | 67.32 ± 3.42 | 51.5 ± 1.2 |
Brice
此外,还研究了Ni-Ti-Cu合金对HUVECs的作用,从而评价其是否具有促血管内皮化功能。利用细胞增殖实验评估了Ni-Ti合金中添加Cu对HUVECs增殖的影响。实验结果表明,随着培养时间的延长,Ni-Ti合金的OD由1 d的1.40增加至3 d的2.27,相对增长率62.14%。Ni-Ti-Cu合金的OD由1 d的1.45增加至3 d的2.53,相对增长率74.48%,所有样品表面上的细胞数目均出现增长,Ni-Ti-Cu合金样品表面的增长更为明显。由此可见,与Ni-Ti合金相比,Ni-Ti-Cu合金促进内皮细胞增殖的作用更强,并且无细胞毒性。
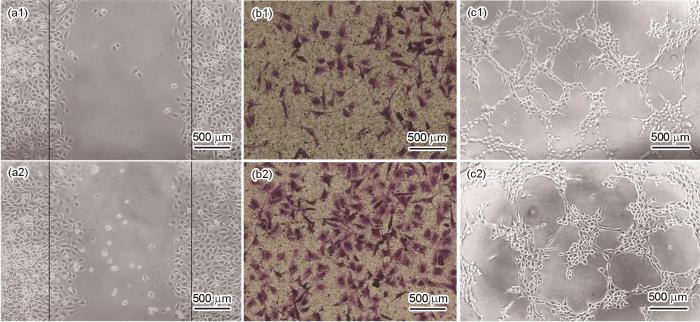
随后,本工作进一步研究Ni-Ti-Cu合金浸提液对内皮细胞迁移能力的影响。通过划痕实验评价不同样品浸提液对单层细胞长满后的横向迁移的影响,即对细胞群体运动的影响。采用Transwell实验分析不同样品浸提液对单个细胞迁移能力的影响。图3a1、a2、b1、b2给出了划痕实验与Transwell实验的结果。如图3a1和a2所示,与不同样品浸提液培养后,内皮细胞均迁移至划痕位置之间的空白区域,见图中黑色线所示。内皮细胞的相对迁移面积比率统计结果表明,Ni-Ti合金的相对迁移面积比率为25.83%,Ni-Ti-Cu合金的相对面积迁移比率为34.51%,Ni-Ti-Cu合金浸提液促进了内皮细胞的横向迁移。图3b1和b2的结果显示,Ni-Ti合金浸提液向下室迁移的内皮细胞数目约为35,而Ni-Ti-Cu合金则约为69。因此与Ni-Ti合金相比,内皮细胞与Ni-Ti-Cu合金浸提液共培养后,向Transwell下室迁移的细胞数目明显增多。划痕实验与Transwell实验结果均表明,Cu在Ni-Ti合金中的添加显著增大了内皮细胞的迁移能力。
图3
图3
Ni-Ti和Ni-Ti-Cu合金的体外细胞实验结果
Fig.3
In vitro cell test results of Ni-Ti (a1-c1) and Ni-Ti-Cu (a2-c2) alloys
(a1, a2) scratch (b1, b2) Transwell (c1, c2) tube formation
血管支架植入人体后,血管的快速修复能够减少新生内膜的生成和血小板吸附,进而会降低血栓及支架内再狭窄发生率。因此本工作还研究了Cu的添加对Ni-Ti合金的血管生成能力的影响。由于内皮细胞在基质胶中可以形成类似管腔的结构,因此采用小管生成实验,研究不同材料浸提液对内皮细胞成血管能力的影响,实验结果如图3c1和c2所示。可见,与Ni-Ti合金相比,Ni-Ti-Cu合金浸提液能促进内皮细胞的小管形成。统计结果显示,与Ni-Ti和Ni-Ti-Cu合金浸提液共培养后的内皮细胞形成的毛细血管状结构的节点数分别为134和156,血管长度分别为22.74和25.79 mm。与Ni-Ti-Cu合金浸提液共培养后的内皮细胞形成的毛细血管状结构的节点数及血管长度均明显增多,表明Ni-Ti-Cu合金能够加速内皮细胞形成网络结构。
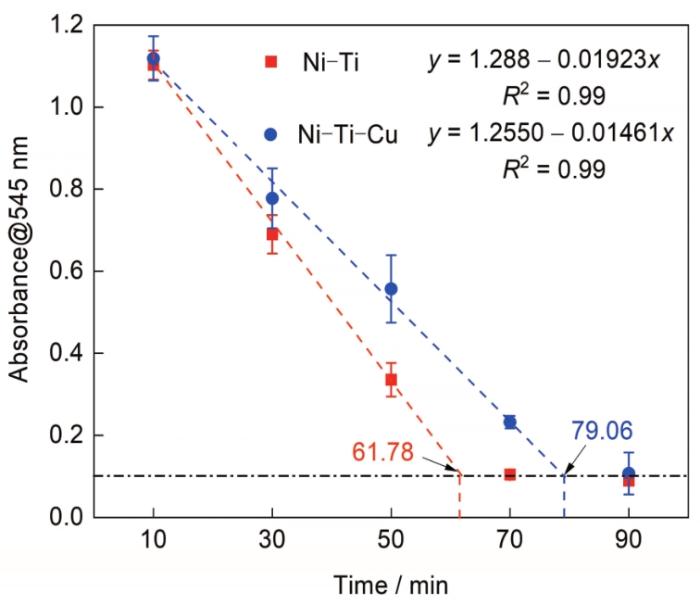
动态凝血实验可以用来评估材料的抗凝血能力。图4为Ni-Ti和Ni-Ti-Cu合金的动态凝血实验结果。可见在相同的凝血时间内,Ni-Ti-Cu合金稀释液的OD更高,说明血液中的血红蛋白更多,即材料表面上凝固的血液更少。进一步对OD与时间进行线性拟合,结果表明,Ni-Ti合金的凝血时间为61.78 min,而Ni-Ti-Cu合金的凝血时间则延长至79.06 min,相比Ni-Ti合金增加近30%,说明Ni-Ti-Cu合金能够显著降低血液凝固速率,具有更优的抗凝血能力。
图4
图4
Ni-Ti和Ni-Ti-Cu合金的动态凝血实验结果
Fig.4
Results of dynamic coagulation experiment of Ni-Ti and Ni-Ti-Cu alloys (y—fitted value of absorbance of residual free hemoglobin in streamer solution measured at 545 nm wavelength, x—dynamic coagulation time, R2—fitting correlation coefficient)
以上实验结果表明,Ni-Ti-Cu合金较Ni-Ti合金具有更为优异的促进血管内皮化功能,这与Cu的添加密切相关。已有研究[39,40]表明,Cu可以提高心肌血管中的内皮生长因子(VEGF)水平,因而会促进内皮细胞的增殖与迁移。本工作研究结果证实,在Ni-Ti合金中添加适量Cu可促进HUVECs的增殖和迁移。动态凝血实验结果表明,Ni-Ti-Cu合金的抗凝血能力也优于Ni-Ti合金。这是因为表面自由能较低的表面对血液中某些成分的吸附能力减弱,因此会表现出更优的抗凝血性能[41]。表面能测试结果证明,Cu的添加使Ni-Ti合金的表面自由能下降,由此可见,Ni-Ti-Cu合金具有更为优异的抗凝血作用,因而更有利于降低血栓的发生率。
3 结论
(1) 与Ni-Ti合金相比,Ni-Ti-Cu合金的Ms点提高,使其显微组织由等轴奥氏体晶粒组织向细板条马氏体组织转变。此外,由于添加Cu,Ni-Ti-Cu合金的表面自由能下降。
(2) 在显微组织变化和较低的表面自由能的共同作用下,与Ni-Ti合金相比,Ni-Ti-Cu合金的耐局部腐蚀与均匀腐蚀能力均得到提高。
(3) Ni-Ti-Cu合金具有更优的促进人脐静脉内皮细胞增殖、迁移和成血管的能力,因而促内皮化作用更强。与Ni-Ti合金相比,Ni-Ti-Cu合金显著降低血液凝固速率,具有更优的抗凝血能力。
参考文献
Shape memory alloys: Metallurgy, biocompatibility, and biomechanics for neurosurgical applications
[J].SHAPE MEMORY ALLOYS possess distinct dynamic properties with particular applications in neurosurgery. Because of their unique physical characteristics, these materials are finding increasing application where resiliency, conformation, and actuation are needed. Nitinol, the most frequently manufactured shape memory alloy, responds to thermal and mechanical stimuli with remarkable mechanical properties such as shape memory effect, super-elasticity, and high damping capacity. Nitinol has found particular use in the biomedical community because of its excellent fatigue resistance and biocompatibility, with special interest in neurosurgical applications. The properties of nitinol and its diffusionless phase transformations contribute to these unique mechanical capabilities. The features of nitinol, particularly its shape memory effect, super-elasticity, damping capacity, as well as its biocompatibility and biomechanics are discussed herein. Current and future applications of nitinol and other shape memory alloys in endovascular, spinal, and minimally invasive neurosurgery are introduced. An understanding of the metallurgic properties of nitinol provides a foundation for further exploration of its use in neurosurgical implant design.
Mechanical fatigue and fracture of Nitinol
[J].
Designing better cardiovascular stent materials: A learning curve
[J].
NiTi based stent for the treatment of acute urinary retention due to benign prostatic hyperplasia: A preliminary study on NiTi wires and tubes under pure bending
[J].
Self-expandable metallic stenting as a bridge to surgery for malignant colorectal obstruction: Pooled analysis of 426 patients from two prospective multicenter series
[J].Self-expandable metallic stenting (SEMS) for malignant colorectal obstruction (MCO) as a bridge to elective surgery (BTS) is a widely used procedure. The aim of this study was to assess short-term outcomes of SEMS for MCO as BTS.This study analyzed pooled data from BTS patients who were enrolled in two multicenter prospective single-arm observational clinical studies that used different stent types. Both studies were conducted by the Japan Colonic Stent Safe Procedure Research Group (JCSSPRG). The first study evaluated the WallFlex™ colonic stent for BTS or palliative treatment (PAL) from May 2012 to October 2013 and the second evaluated the Niti-S™ colonic stent from October 2013 to May 2014. Fifty-three facilities in Japan participated in the studies. Before each study started, the procedure had been shared with the participating institutions by posting details of the standard methods of SEMS placement on the JCSSPRG website. Patients were followed until discharged after surgery.A total of 723 consecutive patients were enrolled in the two studies. After excluding nine patients, the remaining 714 patients were evaluated as a per-protocol cohort. SEMS placement was performed in 426 patients (312 WallFlex and 114 Niti-S) as BTS and in 288 as PAL. In the 426 BTS patients, the technical success rate was 98.1% (418/426). The clinical success rate was 93.8% (392/418). SEMS-related preoperative complications occurred in 8.5% of patients (36/426), perforations in 1.9% (8/426), and stent migration in 1.2% (5/426). Primary anastomosis was possible in 91.8% of patients (391/426), 3.8% of whom (15/393) had anastomosis leakage. The overall stoma creation rate was 10.6% (45/426). The postoperative complication rate was 16.9% (72/426) and mortality rate was 0.5% (2/426).SEMS placement for MCO as BTS is safe and effective with respect to peri-procedural outcomes. Further investigations are needed to confirm long-term oncological outcomes.
Nitinol stents in the femoropopliteal artery: A mechanical perspective on material, design, and performance
[J].Endovascular stenting has matured into a commonly used treatment for peripheral arterial disease (PAD) due to its minimally invasive nature and associated reductions in short-term morbidity and mortality. The mechanical properties of the superelastic Nitinol alloy have played a major role in the explosion of peripheral artery stenting, with modern stents demonstrating reasonable resilience and durability. Yet in the superficial femoral and popliteal arteries, even the newest generation Nitinol stents continue to demonstrate clinical outcomes that leave significant room for improvement. Restenosis and progression of native arterial disease often lead to recurrence of symptoms and reinterventions that increase morbidity and health care expenditures. One of the main factors thought to be associated with stent failure in the femoropopliteal artery (FPA) is the unique and highly dynamic mechanical environment of the lower limb. Clinical and experimental data demonstrate that the FPA undergoes significant deformations with limb flexion. It is hypothesized that the inability of many existing stent designs to conform to these deformations likely plays a role in reconstruction failure, as repetitive movements of the leg and thigh combine with mechanical mismatch between the artery and the stent and result in mechanical damage to both the artery and the stent. In this review we will identify challenges and provide a mechanical perspective of FPA stenting, and then discuss current research directions with promise to provide a better understanding of Nitinol, specific features of stent design, and improved characterization of the biomechanical environment of the FPA to facilitate development of better stents for patients with PAD.
A potential strategy for in-stent restenosis: Inhibition of migration and proliferation of vascular smooth muscle cells by Cu ion
[J].
Electrochemical properties of Ni47Ti49Co4 shape memory alloy in artificial urine for urological implant
[J].
Investigation of surface endothelialization on biomedical nitinol (NiTi) alloy: Effects of surface micropatterning combined with plasma nanocoatings
[J].Plasma nanocoated films with trimethylsilane-oxygen monomers showed outstanding biocompatibility in our previous studies. In this study, endothelialization on biomedical nitinol alloy surfaces was systematically investigated. Our study focuses on elucidating the effects of surface micropatternings with micropores and microgrooves combined with plasma nanocoating. Plasma nanocoatings with controlled thickness between 40 and 50 nm were deposited onto micropatterned nitinol surface in a direct current plasma reactor. Bovine aortic endothelial cells were cultured in vitro on these nitinol samples for 1, 3 and 5 days. It was found that rougher surfaces could enhance cell adhesion compared with the smoother surfaces; the surfaces patterned with micropores showed much more endothelialization than microgrooved surface after a 3 days culture. The cell culture results also showed that plasma nanocoatings significantly further increased cell proliferation and cell adhesion on the micropatterned nitinol surfaces, as compared with non-plasma nanocoated surface of nitinol samples. The surface micropatternings combined with plasma nanocoatings could improve the cell adhesion and accelerate surface endothelialization after implantation of intravascular stents, which is expected to reduce in-stent restenosis.
Evaluation of electrospun PLLA/PEGDMA polymer coatings for vascular stent material
[J].The field of percutaneous coronary intervention has seen a plethora of advances over the past few decades, which have allowed for its development into safe and effective treatments for patients suffering from cardiovascular diseases. However, stent thrombosis and in-stent restenosis remain clinically significant problems. Herein, we describe the synthesis and characterization of fibrous polymer coatings on stent material nitinol, in the hopes of developing a more suitable stent surface to enhance re-endothelialization. Electrospinning technique was used to fabricate polyethylene glycol dimethacrylate/poly l-lactide acid (PEGDMA/PLLA) blend fiber substrate with tunable elasticity and hydrophilicity for use as coatings. Attachment of platelets and arterial smooth muscle cells (SMC) onto the coatings as well as the secretory effect of mesenchymal stem cells cultured on the coatings on the proliferation and migration of arterial endothelial cells and SMCs were assessed. It was demonstrated that electrospun PEGDMA/PLLA coating with 1:1 ratio of the components on the nitinol stent-reduced platelet and SMC attachment and increased stem cell secretory factors that enhance endothelial proliferation. We therefore postulate that the fibrous coating surface would possess enhanced biological compatibility of nitinol stents and hold the potential in preventing stent failure through restenosis and thrombosis.
Synergistic effect of anti-platelet and anti-inflammation of drug-coated Co-Cr substrates for prevention of initial in-stent restenosis
[J].
The molecular mechanism for effects of TiN coating on NiTi alloy on endothelial cell function
[J].The aim of this study is to systematically investigate the molecular mechanism of different effects of nickel titanium (NiTi) alloy surface and titanium nitride (TiN) coating on endothelial cell function. Release of nickel (Ni) ion from bare and TiN-coated NiTi alloys and proliferation of endothelial cells on the two materials were evaluated, and then influence of the two materials on cellular protein expression profiles was investigated by proteomic technology. Subsequently, proteomic data were analyzed with bioinformatics analyses and further validated using a series of biological experiments. Results showed that although the two materials did not affect cell proliferation, the Ni ions released from bare NiTi alloy generated inhibition on pathways associated with actin cytoskeleton, focal adhesion, energy metabolism, inflammation, and amino acid metabolism. In comparison, TiN coating not only effectively prevented release of Ni ions from NiTi alloy, but also promoted actin cytoskeleton and focal adhesion formation, increased energy metabolism, enhanced regulation of inflammation, and promoted amino acid metabolism. Furthermore, the two processes, "the initial mediation of adsorbed serum protein layer to endothelial cell adhesion and growth on the two materials" from our previous study, and "the following action of the two materials on cellular protein expression profile", were linked up and comprehensively analyzed. It was found that in stage of cell adhesion (within 4 h), release of Ni ions from bare NiTi alloy was very low, and the activation of adsorbed proteins to cell adhesion and growth related biological pathways (such as regulation of actin cytoskeleton, and focal adhesion pathways) was almost as same as TiN-coated NiTi alloy. This indicated that the released Ni ions did not affect the mediation of adsorbed proteins to endothelial cell adhesion. However, in stage of cell growth and proliferation, the release of Ni ions from bare NiTi alloy increased with time and reached a higher level, which inhibited endothelial cell function at molecular level, whereas TiN coating improved endothelial cell function. Copyright © 2014 Elsevier Ltd. All rights reserved.
Regulation of endothelial functionality through direct and immunomodulatory effects by Ni-Ti-O nanospindles on NiTi alloy
[J].
Mechanical stability of the diamond-like carbon film on nitinol vascular stents under cyclic loading
[J].
Biocompatibility and mechanical stability of nanopatterned titanium films on stainless steel vascular stents
[J].Nanoporous ceramic coatings such as titania are promoted to produce drug-free cardiovascular stents with a low risk of in-stent restenosis (ISR) because of their selectivity towards vascular cell proliferation. The brittle coatings applied on stents are prone to cracking because they are subjected to plastic deformation during implantation. This study aims to overcome this problem by using a unique process without refraining from biocompatibility. Accordingly, a titanium film with 1 µm thickness was deposited on 316 LVM stainless-steel sheets using magnetron sputtering. Then, the samples were anodized to produce nanoporous oxide. The nanoporous oxide was removed by ultrasonication, leaving an approximately 500 nm metallic titanium layer with a nanopatterned surface. XPS studies revealed the presence of a 5 nm-thick TiO2 surface layer with a trace amount of fluorinated titanium on nanopatterned surfaces. Oxygen plasma treatment of the nanopatterned surface produced an additional 5 nm-thick fluoride-free oxide layer. The samples did not exhibit any cracking or spallation during plastic deformation. Cell viability studies showed that nanopatterned surfaces stimulate endothelial cell proliferation while reducing the proliferation of smooth muscle cells. Plasma treatment further accelerated the proliferation of endothelial cells. Activation of blood platelets did not occur on oxygen plasma-treated, fluoride-free nanopatterned surfaces. The presented surface treatment method can also be applied to other stent materials such as CoCr, nitinol, and orthopedic implants.
In vitro study of role of trace amount of Cu release from Cu-bearing stainless steel targeting for reduction of in-stent restenosis
[J].
In vitro study of stimulation effect on endothelialization by a copper bearing cobalt alloy
[J].
Effect of copper addition on the superelastic behavior of Ni-Ti shape memory alloys for orthodontic applications
[J].
Microstructural, mechanical and citotoxicity evaluation of different NiTi and NiTiCu shape memory alloys
[J].
Property improvement of TiNi by Cu addition for orthodontics applications
[J].
Electrical resistance and deformation during the stress-assisted two-way memory effect in Ni45Ti50Cu5 alloy
[J].
Effect of copper content on superelasticity characteristics in Ti-Ni and Ti-Ni-Cu alloy wires
[J].
Study of the effect of curing treatment in fabrication of SMA/polymer composites on deformational behavior of NiTi-5at.%Cu SMA wires
[J].
Design and development of novel antibacterial Ti-Ni-Cu shape memory alloys for biomedical application
[J].In the case of medical implants, foreign materials are preferential sites for bacterial adhesion and microbial contamination, which can lead to the development of prosthetic infections. Commercially biomedical TiNi shape memory alloys are the most commonly used materials for permanent implants in contact with bone and dental, and the prevention of infections of TiNi biomedical shape memory alloys in clinical cases is therefore a crucial challenge for orthopaedic and dental surgeons. In the present study, copper has been chosen as the alloying element for design and development novel ternary biomedical Ti-Ni-Cu shape memory alloys with antibacterial properties. The effects of copper alloying element on the microstructure, mechanical properties, corrosion behaviors, cytocompatibility and antibacterial properties of biomedical Ti-Ni-Cu shape memory alloys have been systematically investigated. The results demonstrated that Ti-Ni-Cu alloys have good mechanical properties, and remain the excellent shape memory effects after adding copper alloying element. The corrosion behaviors of Ti-Ni-Cu alloys are better than the commercial biomedical Ti-50.8Ni alloys. The Ti-Ni-Cu alloys exhibit excellent antibacterial properties while maintaining the good cytocompatibility, which would further guarantee the potential application of Ti-Ni-Cu alloys as future biomedical implants and devices without inducing bacterial infections.
Corrosion behavior of shape memory, superelastic, and nonsuperelastic nickel-titanium-based orthodontic wires at various temperatures
[J].Nickel-titanium orthodontic wires have various temperature-dependent phases. The purpose of this study was to investigate temperature-dependent corrosion characteristics of shape memory, superelastic, and nonsuperelastic orthodontic wires.Four orthodontic wires were investigated: 27 and 40 degrees C copper Ni-Ti (superelastic and shape memory, respectively), superelastic Ni-Ti, and nonsuperelastic Nitinol Classic. Differential scanning calorimetry (DSC) was used to confirm phase/temperature behavior of the wires. Sectioned halves of as-received archwires were assessed electrochemically in artificial saliva at 5, 24, 37, and 45 degrees C. Open circuit potential (OCP) was monitored for 2h followed by polarization resistance and cyclic polarization tests.DSC results showed Nitinol was primarily martensitic-stable whereas NiTi, 27 degrees C CuNiTi, and 40 degrees C CuNiTi possessed austenite-finish temperatures of approximately 19, 21, and 38 degrees C. The OCP of the CuNiTi wires was significantly greater than NiTi and Nitinol but no apparent trend in values was apparent with regard to temperature or phases present. Corrosion current density (i(corr)) increased with temperature for all wires, but not all were equally influenced. The two lowest austenite-finish temperature wires (27 degrees C CuNiTi and NiTi) approximately tripled in i(corr) from 37 to 45 degrees C. Greater incidence of pitting was observed in the CuNiTi wires.This study showed the corrosion rate of various nickel-titanium wires increase with temperature and different phases present may influence corrosion rate trends.
An electrochemical study of the crevice corrosion resistance of NiTi in Hanks' solution
[J].
Effect of ternary element addition on the corrosion behaviour of NiTi shape memory alloys
[J].
Influence of Cu addition and autoclave sterilization on corrosion resistance and biocompatibility of NiTi for orthodontics applications
[J].
Effect of copper on the localized corrosion resistance of Ni-Ti shape memory alloy
[J].An electrochemical study aiming at the evaluation of corrosion parameters using potentiodynamic and potentiostatic techniques ("scratch" and modified ASTM F746) was conducted in 0.9% NaCl on wires from equiatomic Ni-Ti and ternary Ni44Ti51Cu5 superelastic alloys with Ti90Mo10 as a reference material. The results obtained using potentiostatic tests, that simulate better the behavior of material in service conditions than potentiodynamic ones, indicate that both Ni-Ti and Ni-Ti-Cu wires exhibit low corrosion potentials (approximately 50-150 mV versus SCE) inferior to that of Ti-Mo alloy. The latter proved to be immune to localized corrosion attacks up to 800 mV.
The effect of copper alloying element on the corrosion characteristics of Ti-Ni and ternary Ni-Ti-Cu meltspun shape memory alloy ribbons in 0.9% NaCl solution
[J].
Study of TiCuN/ZrN multilayer coatings with adjustable combination properties deposited on TiCu alloy
[J].
Evaluation of passive oxide layer formation-biocompatibility relationship in NiTi shape memory alloys: Geometry and body location dependency
[J].
Study on biological functions of Cu-bearing stainless steel for urethral system
[D].
含铜不锈钢在泌尿系统中的生物医学功能研究
[D].
Study of in vitro anticoagulant property of the La added medical 316L stainless steel
[J].The in vitro anticoagulant property of a medical grade 316L stainless steel with 0.05% La addition was systematically studied. The results showed that, compared with the conventionally used medical 316L stainless steel, the La added 316L steel possesses less platelets adhesion and less activation of the platelets, and their kinetic clotting time and the plasma recalcification time become longer, which reveals that the activation extent of the latter on intrinsic coagulation factors is smaller than the former and the anticoagulant property of the latter is better. Through measurement of the contact angles of the steels, and calculations of the surface tension of the steels and the interfacial tension between the steels and the blood, the anticoagulant mechanism of the steels was analyzed from the viewpoint of surface energy.
含La医用316L不锈钢的体外抗凝血性能研究
[J].系统研究了在医用316L不锈钢中加入0.05%La后的体外抗凝血性能. 结果表明, 与传统医用316L不锈钢相比, 含La医用316L不锈钢较少地粘附和激活血小板, 且动态凝血时间及血浆复钙时间均延长, 表明其对内源性凝血因子的激活程度下降, 抗凝血性能提高. 通过测量材料的接触角, 计算了表面张力及材料与血液之间的界面张力, 从表面能的角度分析了材料的抗凝血机理.
Surface wettability of water and blood on diversified nanocone‐shaped ZnO films modified with n‐dodecyl mercaptan
[J].
Study on corrosion behavior of the selective laser melted NiTi alloy with superior tensile property and shape memory effect
[J].
Influence of the microstructure on electrochemical corrosion and nickel release in NiTi orthodontic archwires
[J].
Microstructure and corrosion behavior of laser processed NiTi alloy
[J].
Effect of copper-doped titanium nitride coating on angiogenesis
[J].
Evaluation of promoting effect of a novel Cu-bearing metal stent on endothelialization process from in vitro and in vivo studies
[J].Drug eluting stents (DES) have been extensively applied nowadays and reduce the incidence of instent restenosis (ISR) greatly as compared with bare metal stents (BMS). However, the development of DES is hindered by the risk of late stent thrombosis (LST) due to delayed re-endothelialization, while endothelialization is an important process related to ISR and LST after implantation. 316L is a traditional stent material without bioactivity and have a high risk of ISR. Cu is recognized for angiogenesis stimulation in these years. Hence a copper bearing 316L stainless steel (316L-Cu) was prepared and evaluated about its effect on endothelialization in this paper. Compared with traditional 316L, it was proved that 316L-Cu increased the proliferation of co-cultured human umbilical vein endothelial cells (HUVECs) at first day. Moreover, HUVECs stretched better on the surface of 316L-Cu. It also improved the expression of angiogenesis related genes and tube formation ability in vitro. 316L-CuBMS, DES and 316L-BMS were implanted in swine to evaluate the re-endothelialization ability in vivo. And 316L-Cu-BMS showed the best effect on endothelialization with good biosafety. Consequently, 316L-Cu is a kind of promising BMS material for coronary field.