Pb是湿法冶金阳极、蓄电池板栅、防辐射等的重要材料[1 ~6 ] 。为了满足强度、耐蚀、导电等性能需求,工业上常用铅基合金,如湿法冶金中使用Pb-(0.8~1.2)Ag (质量分数,%,下同)合金作为阳极材料,蓄电池中使用Pb-Sb和Pb-Ca合金作为板栅材料等。Al密度低、导电率高、力学性能好,在改善铅合金性能方面具有很大的潜力[7 ] 。当以纳/微米粒子的形式均匀弥散地分布于Pb基体中时,Al可以提高铅基合金的导电性及强度,改善铅基合金的使用性能。研究结果[8 ~10 ] 已证明:原位Al粒子Pb-Al合金是一种潜在的高性能、低成本的湿法冶金阳极或铅蓄电池板栅材料;添加0.01~0.02的Al即可显著提高Pb-Ca合金耐蚀性;添加0.11的Al即能显著提高锌湿法冶金阳极合金Pb-Ag的强度和耐蚀性等。
然而,Pb-Al合金相图中存在范围很宽的液-液两相区及液-固两相区,凝固过程中首先发生液-液/液-固分相——弥散相液滴/粒子自熔体沉淀析出,极易形成弥散相尺寸粗大或相偏析严重的凝固组织[11 ~13 ] ,其凝固过程研究和工业制备受到严重限制。本工作以Pb-0.15Al合金为研究对象,实验研究定向凝固条件下合金的液-固分相与凝固行为,并基于群体动力学方法建立液-固分相合金定向凝固模型,实验与模拟相结合,探索Pb-Al合金液-固分相过程与原位粒子复合凝固组织的形成规律。
1 实验方法
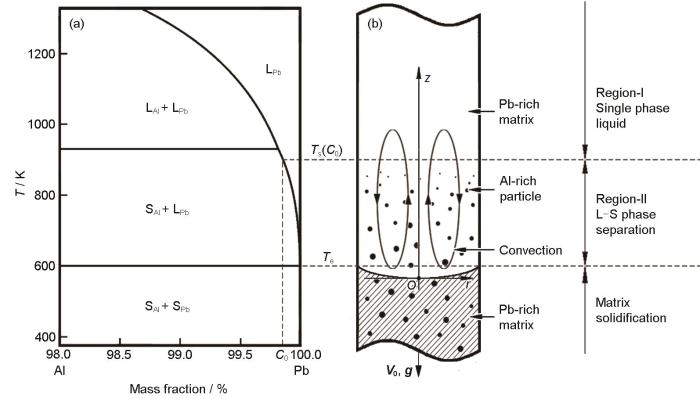
以纯度为99.99%的Pb和Al为原料,配制Pb-0.15Al合金(合金初始成分(C 0 ))。图1 为Pb-Al合金局部相图及定向凝固条件下Pb-Al合金液-固分相过程示意图。实验过程如下:首先将合金原料加入内径4 mm、壁厚1 mm的Al2 O3 坩埚,用电阻炉加热熔化并升温至1073 K (高于合金组元互溶温度约150 K),保温并充分搅拌30 min,确保形成均一熔体;然后,以不同的提拉速率将坩埚下拉至Ga-In-Sn液态金属中进行冷却凝固,形成尺寸为直径4 mm、长100 mm的圆柱形试样。将试样沿轴向切割,经研磨抛光后,使用Inspect F50型扫描电子显微镜(SEM)观察合金的微观组织,采用SISC IAS V8.0定量金相分析软件统计富Al粒子的尺寸。实验过程中,采用直径0.1 mm的W-Re热电偶测定合金熔体冷却凝固过程中试样中心的温度,使用XSR90-04V0型温度记录仪记录冷却曲线。
图1
图1
Pb-Al合金局部相图及定向凝固条件下Pb-Al合金液-固分相过程示意图
Fig.1
Pb-rich part of the Pb-Al phase diagram (a) and the liquid-solid phase separation process in a directionally solidifying Pb-Al alloy (b) (T —temperature, LAl —Al-rich liquid phase, SAl —Al-rich solid phase, LPb —Pb-rich liquid phase, SPb —Pb-rich solid phase, C 0 —initial composition of the alloy, T s (C 0 )—solubility line temperature of initial composition alloy, T e —eutectic reaction temperature of Al-Pb alloy, rOz —coordinate system, V 0 —pulling velocity of samples, g
2 实验结果
图2 给出了Pb-0.15Al合金以不同凝固速率定向凝固时试样凝固界面前沿中心轴线的温度分布曲线。可见,随着凝固速率的增大,凝固界面前沿合金熔体内的轴向温度梯度逐渐减小。
图2
图2
不同凝固速率条件下试样凝固界面前沿中心轴线处的温度分布曲线
Fig.2
Experimental (symbols) and calculated (lines) results of the temperature profile in front of the solidification interface along the central z -axes for the Pb-0.15Al alloy solidified at different solidification rates (V 0 )
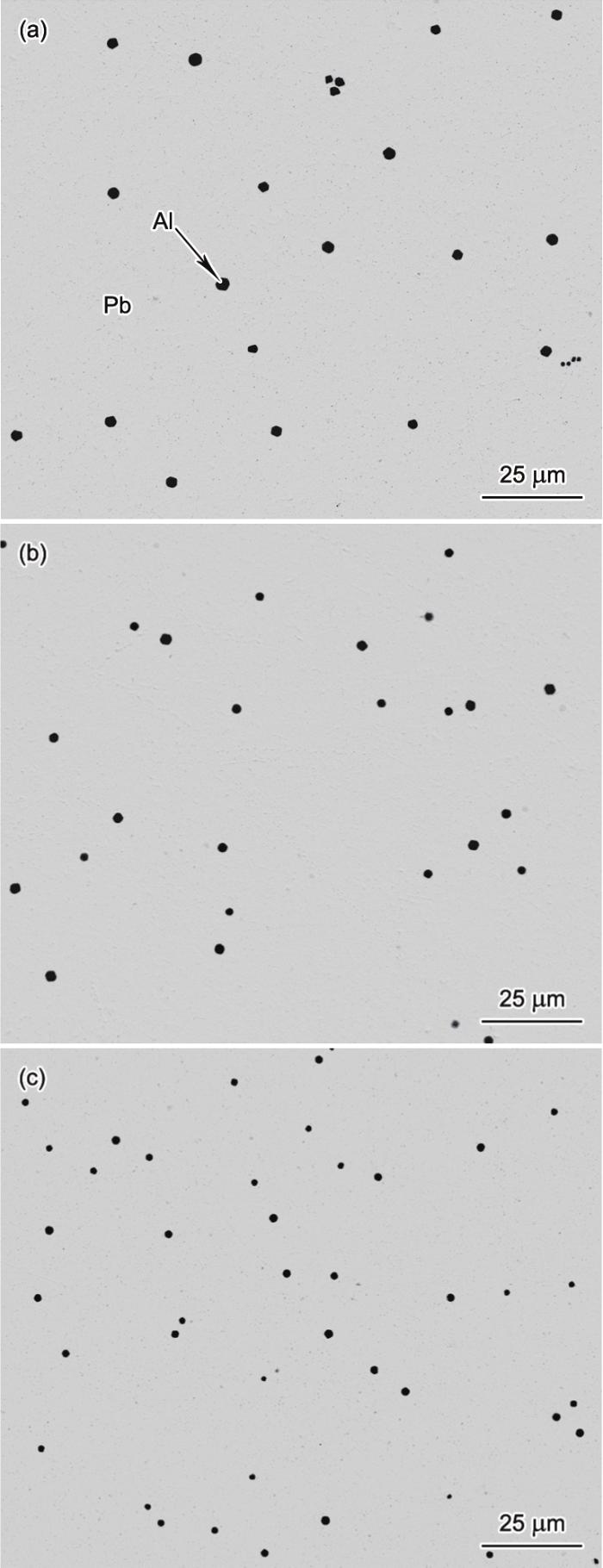
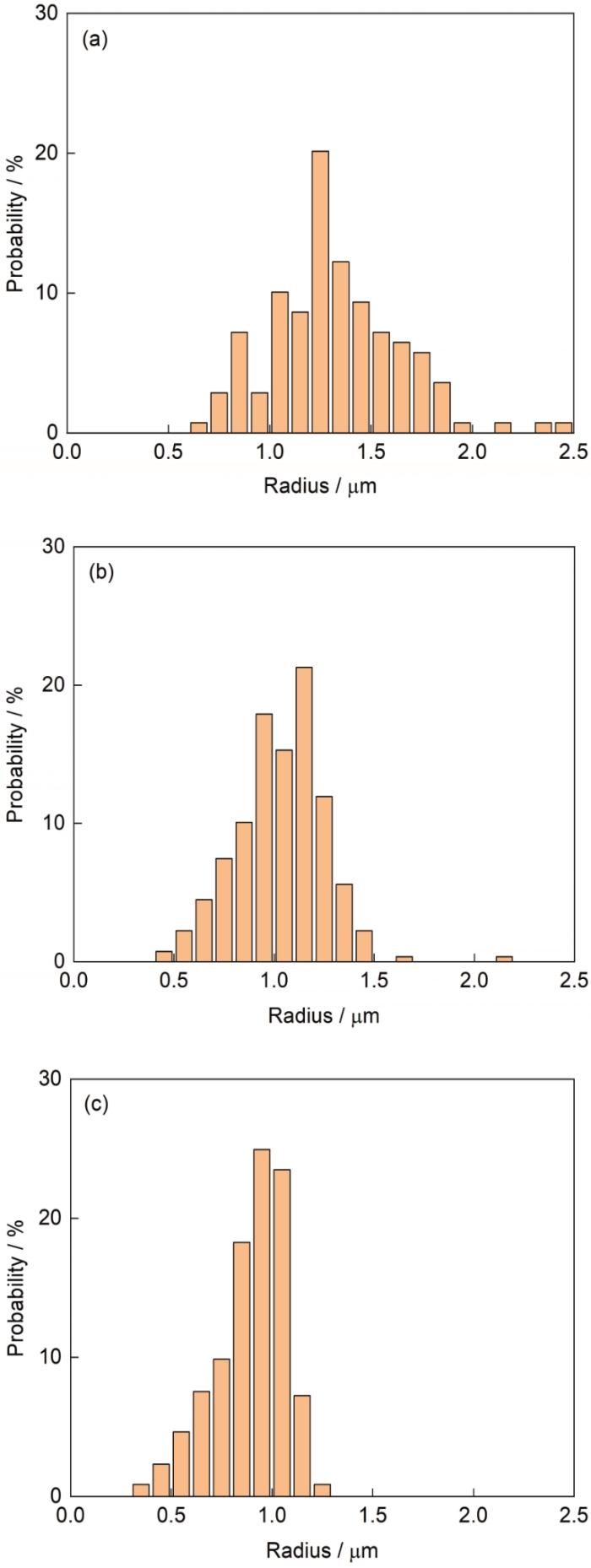
图3 给出了不同凝固速率下Pb-0.15Al合金定向凝固后的微观组织,其中浅色的基体为Pb相,深色的粒子为富Al相。富Al粒子为近球形,均匀分布于Pb基体中。随着凝固速率的增大,富Al粒子的尺寸减小,数量密度增大。图4 给出了不同凝固速率下Pb-0.15Al合金中富Al粒子的二维尺寸分布。可见,富Al粒子尺寸呈单峰分布,且随着凝固速率的增大,尺寸分布曲线峰值变高、宽度变窄并向左偏移,粒子平均半径下降(图5 )。
图3
图3
以不同凝固速率定向凝固时Pb-0.15Al合金的微观组织
Fig.3
Microstructures of the Pb-0.15Al alloy direction-ally solidified at V 0 = 4 mm/s (a), 7 mm/s (b), and 10 mm/s (c)
图4
图4
不同凝固速率下Pb-0.15Al合金中富Al粒子的二维半径分布
Fig.4
2D radius distributions of the Al-rich particles in the Pb-0.15Al alloy directionally solidified at V 0 = 4 mm/s (a), 7 mm/s (b), and 10 mm/s (c)
图5
图5
Pb-0.15Al合金中富Al粒子的二维平均半径(<R >2D )随凝固速率的变化
Fig.5
Average 2D radius (<R >2D ) of the Al-rich particles in the Pb-0.15Al alloys directionally solidified at different V 0
3 分析讨论
3.1 液- 固分相过程模型与模拟方法
当均一的Pb-Al合金熔体冷却进入液-固分相温度区间以后,弥散相富Al粒子自熔体中沉淀析出,其形核率(I )可用下式计算[14 ,15 ] :
I = N 0 ∙ 4 n c 2 3 ⋅ 6 D λ ⋅ Δ G c 3 π k B T n c 2 1 2 ⋅ e x p - Δ G c k B T (1)
式中,N 0 为单位体积基体熔体中的原子数; n c R c = 2 σ / Δ G V σ Δ G V D 为Al在Pb中的扩散系数;λ 为Al原子的平均跳跃距离; k B T 为热力学温度; Δ G c 16 π σ 3 / [ 3 ( Δ G V ) 2 ]
富Al粒子形核后,将在过饱和熔体中扩散长大,长大速率(v R )为[16 ,17 ] :
v R = D C m - C I m C β - C I m 1 R + R 3 d C β d T d T d t C β - C I m (2)
式中, C m C β C I m C e m ⋅ e x p ( α s / R ) C e m α s ( 2 σ Ω β ) / ( k B T ) Ω β R 的富Al粒子与基体熔体界面处熔体中溶质Al的摩尔浓度;t 为时间。
为了定量地描述合金液-固分相凝固过程中原位富Al粒子复合凝固组织的形成,定义富Al粒子的半径分布函数 f ( r , z , R , t ) f ( r , z , R , t ) d R t ( r , z ) R R + d R f [18 ,19 ] :
∂ f ∂ t + ∇ ⋅ u S + V f + ∂ ∂ R v R f = ∂ I ∂ R | R = R c (3)
式中, u S 2 g ( ρ β - ρ m ) R 2 / ( 9 η m ) g ρ β ρ m η m 为基体熔体黏度),为富Al粒子的Stokes运动速度[20 ] ; V V 0 + V c V 0 V c V 0 V 0 rOz 坐标系(图1 b,O 为稳态凝固条件下凝固界面的最低点)中分解: u S u S z j V V r i + V z j V 0 V 0 z j V c V c r i + V c z j i j r 、z 方向的单位向量; u S z V r V z V 0 z V c r V c z r 或z 方向的分量,其正负表示方向)。
∇ ⋅ ρ V = 0 (4)
∂ ρ V ∂ t + ρ V ⋅ ∇ V = - ∇ p + ∇ 2 η V - 4 π 3 ( u S + V ) ⋅ ∇ ∫ 0 ∞ [ ρ β ( u S + V ) f ] R 3 d R (5)
式中,ρ 为合金熔体(基体熔体与富Al粒子的两相混合物)的密度; p η )采用Einstein黏度公式计算[22 ] :η = η m (1 + 2.5ϕ ) (其中,ϕ 为合金熔体内富Al粒子的体积分数)。
合金凝固过程中,热量通过导热、对流进行传输,且受熔体中富Al粒子空间迁移的影响。合金的温度场满足如下方程[13 ] :
∂ ( ρ c p T ) ∂ t + ∇ ⋅ V ρ c p T = ∇ 2 k T - 4 π 3 ⋅
∇ ⋅ ∫ 0 ∞ u S + V ρ β c p β - ρ m c p m f T R 3 d R + Q s / l (6)
- k c r u ∂ T ∂ r | r = r s c , z > z G a = h 1 T s c - T o v e n (7)
- k c r u ∂ T ∂ r | r = r s c , z < z G a = h 2 T s c - T G a (8)
式中, c p c p β c p m k 和 k c r u r s c z Ga 为Ga-In-Sn液态金属的上表面位置; h 1 h 2 T s c T o v e n T G a Q s/l = ρ m LV 0 (1 - ϕ ) (其中, L
溶质传输由扩散、对流和富Al粒子的运动控制。浓度场满足如下控制方程:
∂ C m i x ∂ t + ∇ ⋅ C m i x V = ∇ 2 [ D ( C m - C e m ) 1 - ϕ ] - 4 π 3 ∇ ⋅ ∫ 0 ∞ u S + V C β - C m f R 3 d R (9)
式中,C mix = (1 - ϕ )C m + ϕCβ ,为合金熔体内Al的摩尔浓度。
采用有限体积法将以上流场、温度场、浓度场和富Al粒子半径分布函数控制方程离散,并与合金相图[23 ] 进行耦合求解,即可模拟定向凝固条件下液-固分相Pb-Al合金凝固组织形成过程。
模拟计算中使用的参数见表1 [24 ] ,用拟合法确定 h 1 、 h 2 σ σ ) ,选取不同的 h 1 、 h 2 h 1 h 2 2 ·K),进而,使用该有效对流换热系数模拟计算其他凝固速率下合金熔体中心轴线处的温度分布曲线,通过检查实测值与计算结果之间的符合程度(图2 ),进一步验证拟合所得参数值的正确性;然后,基于上述已知参数,选取不同的σ 模拟计算4 mm/s凝固速率时合金凝固组织中富Al粒子的平均半径,通过检查实测值与计算结果之间的符合程度,确定σ = 0.135 J/m2 ,进而,使用该σ 模拟计算其他凝固速率下合金凝固组织中富Al粒子的平均半径,通过检查实测值与计算结果之间的符合程度(图5 ),进一步验证所取σ 的正确性。
3.2 液- 固分相Pb-Al 合金凝固组织形成过程
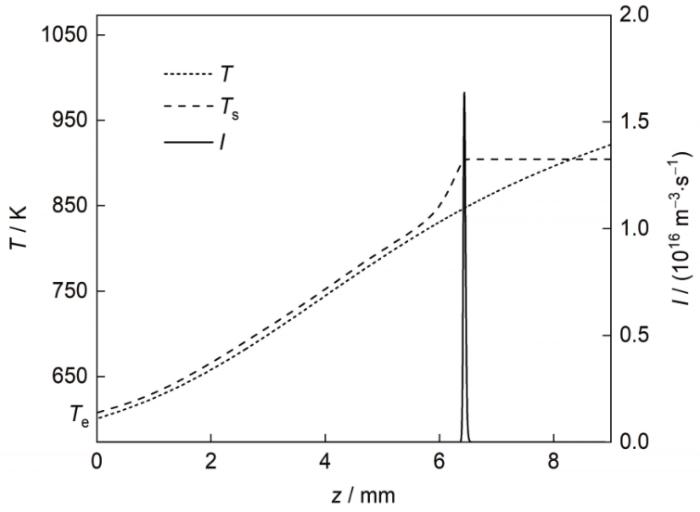
当液-固分相Pb-Al合金的定向凝固过程进入稳定凝固状态以后,凝固界面前沿的合金熔体可分为如图1 b所示的2个区域,即均一熔体区和液-固分相区。图6 给出凝固速率为4 mm/s时液-固分相区内富Al粒子的形核过程。可见,当均一合金熔体向下运动进入液-固分相区时,熔体温度(T )降至合金组元互溶温度(T s )以下,熔体中Al的浓度高于Al在Pb熔体中的溶解度,即熔体处于过饱和(或过冷)状态;此后,随着熔体继续向下移动,熔体过冷度(ΔT = T s - T )逐渐增大。当ΔT 增至某一值时,富Al粒子开始从基体熔体中形核析出。由于粒子的形核及随后的扩散长大消耗熔体中的溶质,导致T s 降低、ΔT 减小,所以富Al粒子的形核过程仅持续很短的时间、发生在很小的温度范围内;最大形核率位置与最大过冷度位置相对应。
图6
图6
凝固速率为4 mm/s时熔体温度(T )、平衡组元互溶温度(T s )及粒子形核率(I )沿中心轴线的变化
Fig.6
T , solubility line temperature (T s ) of the matrix melt, and nucleation rate (I ) of the Al-rich particles in front of the solidification interface along the central z -axes for the Pb-0.15Al alloy solidified at V 0 = 4 mm/s
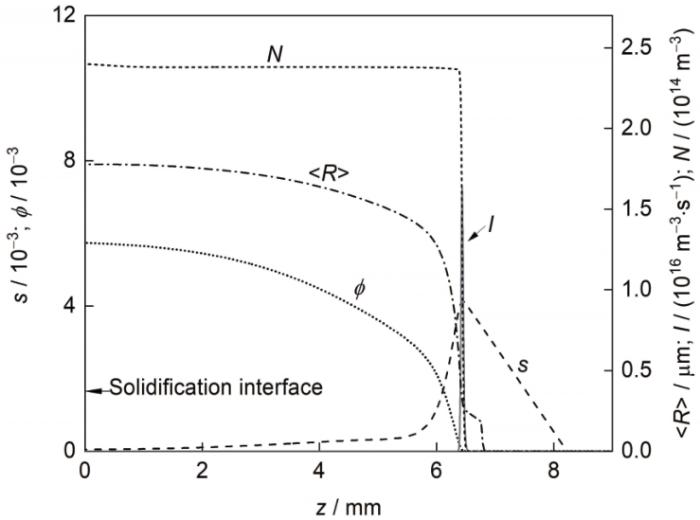
图7 给出了凝固速率为4 mm/s时凝固界面前沿富Al粒子的形核率、数量密度、平均半径、体积分数及熔体过饱和度(s = x - x me ,基体熔体中Al的摩尔分数(x )与平衡摩尔分数(x me )的差值)沿试样中心轴的变化曲线。可见,富Al粒子形核后逐渐向凝固界面迁移,并在迁移过程中进行扩散长大,其平均半径和体积分数逐渐增大;形核过程结束后,熔体的过饱和度不断降低。
图7
图7
凝固速率为4 mm/s时富Al粒子形核率、数量密度(N )、平均半径(<R >)、体积分数(ϕ )及熔体过饱和度(s )沿试样中心轴线的变化
Fig.7
I , number density (N ), average radius (<R >), and volume fraction (ϕ ) of the Al-rich particles, and supersaturation (s ) of the matrix melt in front of the solidification interface along the central z -axes for the Pb-0.15Al alloys solidified at V 0 = 4 mm/s
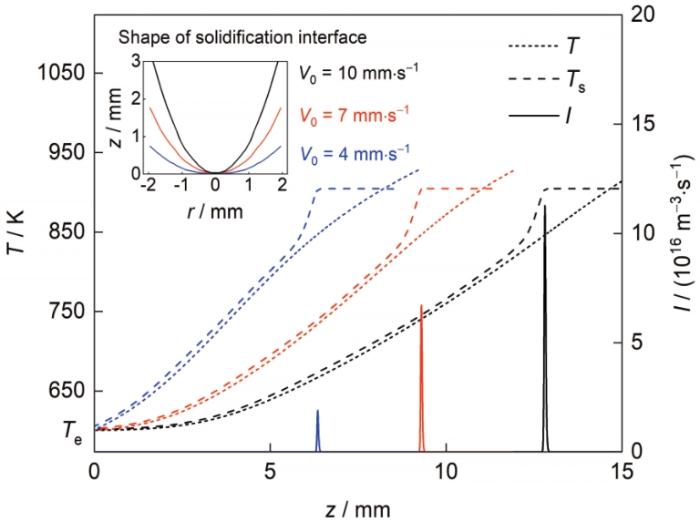
图8 和9 给出了不同凝固速率下熔体温度、组元互溶温度、弥散相粒子形核率、数量密度及平均半径沿z 轴的变化。可见,随着凝固速率的增大,液-固分相区内熔体的轴向温度梯度减小,尤其是凝固界面前几个毫米的范围内,由于凝固潜热的释放,合金熔体更倾向于径向冷却(凝固界面形状见图8 插图)。但形核位置处熔体的冷却速率却一直随着凝固速率的增大而增大:计算结果表明,对应于4、7和10 mm/s 3种凝固速率,试样中心轴线上富Al粒子形核位置处合金熔体的冷却速率分别约为126、220和275 K/s。因此,随着凝固速率的增大,富Al粒子的形核率及数量密度增大、平均半径减小,即提高凝固速率可以细化富Al粒子,提高其弥散度,有利于获得均匀弥散的凝固组织。
图8
图8
不同凝固速率下熔体温度、组元互溶温度及粒子形核率沿z 轴的变化,以及凝固界面形状
Fig.8
T , T s of the matrix melt, and I of the Al-rich particles in front of the solidification interface along the central z -axes for the Pb-0.15Al alloys solidified at V 0 = 4 mm/s (blue lines), 7 mm/s (red lines), and 10 mm/s (black lines) (The inset shows the shape of the solidification interface at different V 0 )
图9
图9
不同凝固速率下富Al粒子形核率、数量密度及平均半径沿z 轴的变化
Fig.9
I , N , and <R > of the Al-rich particles in front of the solidification interface along the central z -axes for the Pb-0.15Al alloys solidified at V 0 = 4 mm/s (blue lines) and 10 mm/s (black lines)
3.3 富Al 粒子Stokes 运动对凝固组织形成过程的影响
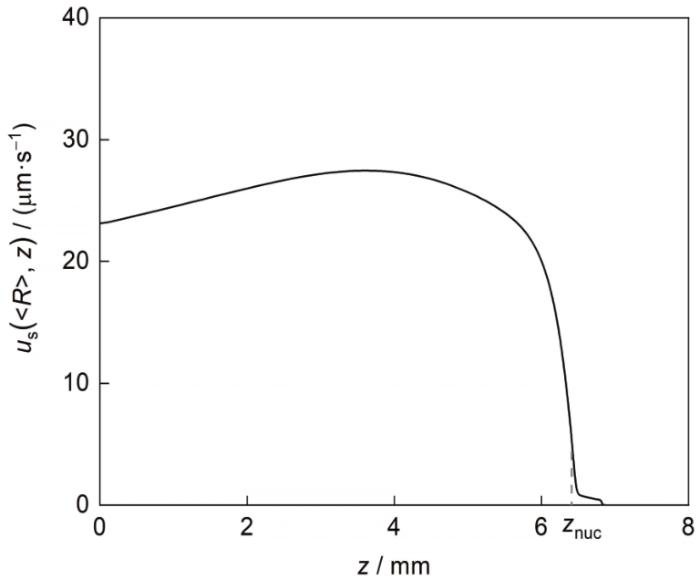
Al的密度远小于Pb,富Al粒子Stokes运动的方向为沿z 轴向上(u S z 图10 给出了凝固速率为4 mm/s时合金凝固界面前沿中心轴线处平均尺寸富Al粒子的Stokes运动速率 u S ( < R > , z ) z 的变化关系。 u S ( < R > , z ) < R > 2 η m z 减小), < R > η m u S ( < R > , z ) z 的减小而先增大后减小的趋势,如图10 所示。计算表明,凝固速率为4 mm/s时, u S ( < R > , z )
图10
图10
凝固速率为4 mm/s时凝固界面前沿中心轴线处平均尺寸富Al粒子的Stokes运动速率(u S (<R >, z ))沿z 轴的变化
Fig.10
Stokes movement rate of the average size Al-rich particles (u S (<R >, z )) in front of the solidification interface along the central z -axes for the Pb-0.15Al alloy solidified at V 0 = 4 mm/s (z nuc —the axial position where Al-rich particles have just finished nucleation)
图11 给出了凝固速率为4 mm/s时凝固界面前沿中心轴线处富Al粒子数量密度与体积分数沿z 轴的变化。可见,富Al粒子的Stokes运动会改变熔体内粒子的空间分布。分析如下:在不考虑熔体对流影响条件下,当凝固进入稳态(∂ f ∂ t ∂ I ∂ R | R = R c 式(3)可得:
N z = N z n u c - 1 V 0 z ∫ 0 ∞ ( u S z f ) z d R (10)
ϕ ( z ) = ϕ D ( z ) - 4 π 3 V 0 z ∫ 0 ∞ ( R 3 u S z f ) z d R (11)
式中,z nuc 为粒子形核结束位置,z < z nuc ;N (z nuc )为z nuc 处合金熔体内粒子的数量密度;ϕ D (z )为纯扩散长大条件下粒子的体积分数变化,随z 的减小而增大;(u S z fz R 3 u S z f ) z z 处的值。
图11
图11
凝固速率为4 mm/s时凝固界面前沿中心轴线处富Al粒子数量密度与体积分数沿z 轴的变化
Fig.11
N (a) and ϕ (b) of the Al-rich particles in front of the solidification interface along the central z -axes for Pb-0.15Al alloys solidified at V 0 = 4 mm/s ( u S —Stokes movement of Al-rich particles, V c —convective flow of melt. Insets show the high magnified curves)
由 式(10)和(11)可知,若富Al粒子不发生Stokes运动(u S z N (z ) = N (z nuc )、ϕ (z ) = ϕ D (z ),即形核结束后粒子的数量密度保持不变;由于粒子在试样向下运动过程中不断长大,体积分数呈现随z 的减小而增加的趋势。若富Al粒子发生Stokes运动,由于u S z N (z ) > N (z nuc )、ϕ (z ) > ϕ D (z ),即富Al粒子Stokes运动方向与合金凝固方向相同,导致粒子在凝固界面前发生富集。反之,弥散相粒子的Stokes运动方向与凝固方向相反 ( u S z N (z ) < N (z nuc )、ϕ (z ) < ϕ D (z ))。弥散相粒子富集或贫化的程度与|u S (<R >) / V 0 |正相关。应该指出,稳态凝固条件下,凝固界面前沿弥散相粒子的富集或贫化并不会导致凝固组织中弥散相分布的宏观偏析。凝固组织中弥散相粒子的数量密度和体积分数分别满足N (z ) = N (z nuc )、ϕ (z ) = ϕ D (0),与轴向位置无关。
3.4 熔体对流对凝固组织形成过程的影响
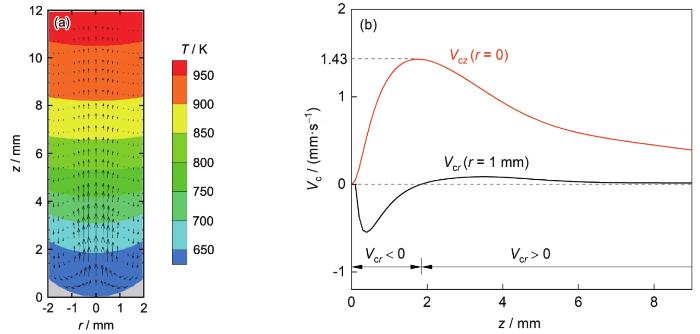
熔体对流是富Al粒子Stokes运动和熔体内温度梯度共同作用的结果。对于Pb-0.15Al合金而言,富Al粒子的体积分数很小,其迁移运动对熔体对流的影响很弱,熔体对流主要由熔体内的温度梯度决定。图12 a给出了凝固速率为4 mm/s时,合金稳态凝固条件下凝固界面前沿合金熔体内的温度场和流场。可见,在同一轴向位置处,试样表面附近熔体温度低、密度大、向下流动;试样中心熔体温度高、密度小、向上流动。V c z r = 0)处,V c r r = 1 mm位置处。图12 b给出了V c z r = 0)与V c r r = 1 mm)沿轴向位置z 的变化。由图可知,试样轴向对流较强而径向对流较弱,最大对流速率约为1.43 mm/s。
图12
图12
凝固速率为4 mm/s时凝固界面前沿合金熔体内的温度场和流场,以及熔体对流速度的r 、z 分量(V c r V c z z 的变化
Fig.12
Temperature field and flow field of the melt in front of the solidification interface when solidifying the alloy at V 0 = 4 mm/s (a), z component of the melt convection velocity at the position of r = 0 (V c z r = 0)), and r component of the melt convection velocity at the position of r = 1 mm (V c r r = 1 mm)) along the axial position z (b)
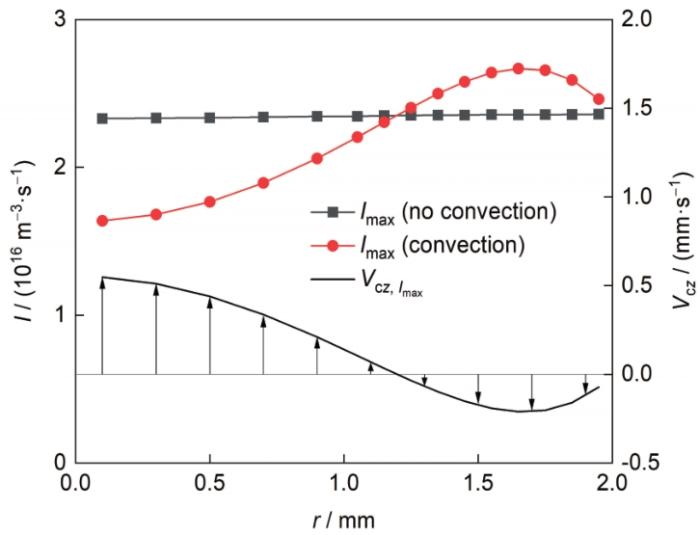
熔体对流对合金的凝固组织形成过程具有重要影响。首先,熔体对流会影响富Al粒子的形核行为。图13 给出了凝固速率为4 mm/s时富Al粒子峰值形核率(I max )及对应位置处熔体对流速度的z 分量(V c z , I m a x ) 沿试样径向的变化。可见,当熔体内无对流运动时,I max 沿试样径向的分布较为均匀。当熔体内存在对流运动时,I max 沿试样径向的分布发生改变:试样中心区域(约r < 1.2 mm),熔体轴向对流速度( V c z r > 1.2 mm), V c z
图13
图13
凝固速率为4 mm/s时富Al粒子峰值形核率(I max )及对应位置处熔体对流速度的z 分量(V c z , I m a x ) 沿试样径向的变化
Fig.13
Maximum nucleation rate (I max ) of the Al-rich particles and the V c z V c z , I m a x ) along the r direction for the sample solidified at V 0 = 4 mm/s
∂ T ∂ t = ∂ T ∂ z ∂ z ∂ t + ∂ T ∂ r ∂ r ∂ t = ∂ T ∂ z V z + ∂ T ∂ r V r (12)
式中, V z V 0 z + V c z V r V c r z ≈ 6.3 mm)附近,| V c r 图12 ),可以忽略,则:
∂ T ∂ t ≈ ∂ T ∂ z V z = ∂ T ∂ z ( V 0 z + V c z ) (13)
在试样中心区域, V c z V 0 V 0 z + V c z V 0 z V c z V 0 V 0 z + V c z V 0 z I m a x
其次,熔体对流会影响凝固界面前沿合金熔体内富Al粒子的空间分布。这是由于,一方面,熔体对流对弥散相粒子形核率的影响直接导致粒子数量密度沿试样径向的不均匀分布,即在试样中心区域,N 较小;试样表面附近,N 较大(图14 )。计算表明,对于直径为4 mm的样品,形核结束时N 沿试样径向分布的相对差值可达28.4%。另一方面,富Al粒子形核结束后,熔体对流还会通过搅拌作用进一步影响其数量密度及体积分数的空间分布,如图11 和14 所示。具体影响机理如下:稳态凝固条件下,单独考虑对流 V c 的影响(忽略粒子Stokes运动 u s ),在凝固界面前沿的非形核区域内,联立 式(3)和(4)并对其积分可得:
N z = N z n u c + 1 V 0 ∫ z z n u c ( V c r ∂ N ∂ r + V c z ∂ N ∂ z ) d z (14)
ϕ ( z ) = ϕ D ( z ) + 1 V 0 ∫ z z n u c ( V c r ∂ ϕ ∂ r + V c z ∂ ϕ ∂ z ) d z (15)
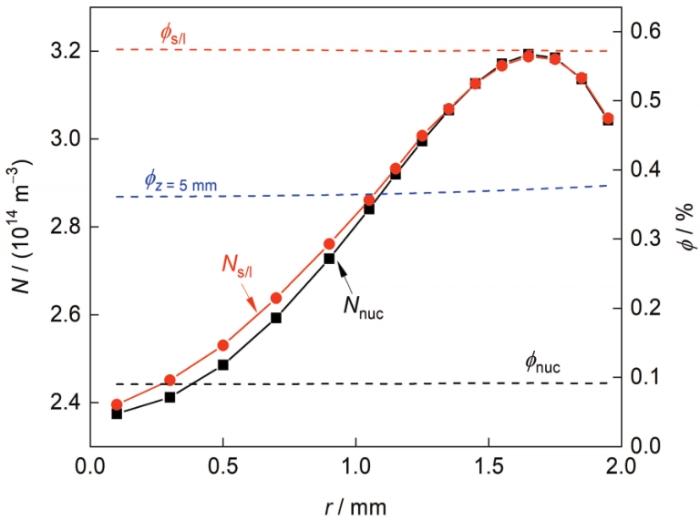
图14
图14
凝固界面前沿合金熔体内富Al粒子数量密度及体积分数沿试样径向分布
Fig.14
N and ϕ of the Al-rich particles in front of the solidification interface along the r direction for the sample solidified at V 0 = 4 mm/s (The subscript “nuc”, “z = 5 mm”, and “s/l” refer to the axial position of z = z nuc , z = 5 mm, and the solidification interface, respectively)
式(14)和(15)表明, V c 对 N ϕ 空间分布的影响既与V c r V c z N 、ϕ 在r 、z 方向上分布的梯度有关。在本实验条件下,凝固界面前沿合金熔体内富Al粒子的数量密度在轴向上分布较为均匀(∂ N / ∂ z ≈ 0 ) ,径向上差异较大(图14 )。所以 V c z N 的分布影响较小,而 V c r N 沿径向的分布趋向于均匀,即:由中心向表面流动的熔体使试样表面附近粒子的数量密度下降;由表面向中心流动的熔体使试样中心区域粒子的数量密度升高。图14 给出了形核结束位置及凝固界面处合金熔体内富Al粒子的数量密度N nuc 和N s/l 沿试样径向的分布情况。可见,随着凝固过程的进行,N 沿试样径向的分布趋向于均匀:粒子数量密度沿试样径向分布的相对差值由28.4%降至27.3%。
凝固界面前沿合金熔体内富Al粒子的体积分数在径向上分布较为均匀(∂ϕ / ∂r ≈ 0,图14 ),轴向上差异较大(由于粒子形核后在向凝固界面迁移过程中发生扩散长大,所以∂ϕ / ∂z < 0,图11 b),因此, V c r ϕ 的分布影响较小,而 V c z 式(15)进行分析,可知:在熔体中心区域,V c z 图13 ),则ϕ (z ) > ϕ D (z ) (图11 b);熔体表面附近,V c z 图13 ),则ϕ (z ) < ϕ D (z )。需要说明的是,在凝固界面处始终满足ϕ (0) = ϕ D (0) (见图11 b,z = 0处绿线与黑线重合)。这是因为在不考虑粒子Stokes运动的条件下,虽然不同位置处弥散相的体积分数不同,但两相混合物的溶质浓度 C m i x ϕ )C m + ϕCβ = C 0 保持不变。
3.5 弥散型凝固组织的形成条件
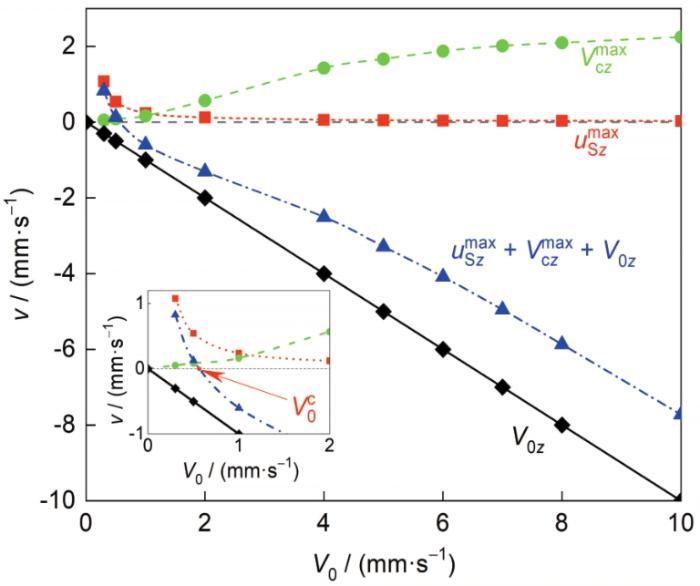
Pb-Al液-固分相合金形成弥散型凝固组织的前提条件是合金的定向凝固过程可以实现稳态凝固,这要求凝固界面前沿合金熔体内所有富Al粒子(任意尺寸、任意位置)始终向着凝固界面迁移[25 ] ,即满足: ( V 0 z + V c z + u S z ) ∀ R , r , z u S z m a x V c z m a x V 0 z 图15 给出了Pb-0.15Al合金定向凝固过程中 u S z m a x V c z m a x u S z m a x V c z m a x V 0 z V 0 的变化。可见,当富Al粒子不发生Stokes运动且熔体内无对流(u S z m a x V c z m a x V 0 z 图15 中黑色线);当熔体内弥散相粒子Stokes运动方向与凝固方向相同(u S z m a x 图15 中红色线)或存在与凝固方向相同的熔体对流(V c z m a x 图15 中绿色线)时,为了使合金实现稳态凝固, V 0 V 0 V 0 z u S z m a x V c z m a x V 0 c 图15 中蓝色线,临界凝固速率 V 0 c
图15
图15
Pb-0.15Al合金定向凝固条件下粒子Stokes运动速度z 分量的最大值(u S z m a x ) 、熔体对流速度z 分量的最大值(V c z m a x ) 及合速度z 分量的最大值(u S z m a x V c z m a x V 0 z ) 随凝固速率的变化
Color online
Fig.15
Maximum of the z component of Stokes movement velocity of Al-rich particles (u S z m a x ) , maximum of the z component of melt convection velocity (V c z m a x ) and maximum of the z component of the resultant velocity (u S z m a x V c z m a x V 0 z ) versus V 0 when directionally solidifying Pb-0.15Al alloys (V 0 c
4 结论
(1) 液-固分相合金定向凝固过程中,凝固界面前沿存在一过冷区,弥散相粒子在峰值过冷度附近形核,并在向凝固界面移动过程中进行扩散长大。随着凝固速率的提高,弥散相粒子形核率升高、数量密度增大、平均半径减小。
(2) 弥散相粒子相对于基体熔体的迁移运动方向与合金凝固方向相同时,弥散相粒子在凝固界面前发生富集,方向相反时,弥散相粒子在凝固界面前发生贫化。稳态凝固条件下,凝固界面前沿弥散相粒子的富集或贫化并不会导致凝固组织中弥散相分布的宏观偏析。
(3) 熔体对流主要通过影响局部熔体的冷却速率影响液-固分相合金的凝固行为和组织,与凝固方向相反的对流提高熔体的冷却速率和弥散相粒子的形核率,与凝固方向相同的对流使熔体冷却速率和粒子形核率降低。熔体对流使得弥散相粒子形核率、数量密度及平均半径沿试样径向呈不均匀分布。
(4) 定向凝固液-固分相合金形成弥散型凝固组织的必要条件为合金的凝固速率足够高,确保凝固界面前沿熔体内所有尺寸粒子均向凝固界面迁移。合金熔体内与凝固方向相同的弥散相粒子Stokes运动和基体熔体对流运动会增大合金实现稳态凝固所需的临界凝固速率,不利于合金稳态凝固的建立和均匀弥散型凝固组织的形成。
参考文献
View Option
[1]
Yi T F Dai C S Hu X G et al Research progress in lead foam grid materials for lead acid batteries
[J]. Batte. Bimonth. , 2006 , 36 : 229
[本文引用: 1]
伊廷锋 , 戴长松 , 胡信国 等 铅酸电池泡沫铅板栅材料的研究进展
[J]. 电池 , 2006 , 36 : 229
[本文引用: 1]
[2]
Xu L Yan M Wang X Y et al The influences of silver and zinc addition on the electrochemical performances of the Pb-Ca-Sn grids for lead acid batteries
[J]. Metall. Res. Technol. , 2018 , 115 : 6
[3]
Wu Z F Hu C Mu J Y et al Investigation of Pb-Sr and Pb-Ca binary alloys as grids for lead-acid batteries
[J]. Int. J. Electrochem. Sci. , 2019 , 14 : 8709
[4]
Wang X K Chen S Chen B M et al Study status of energy saving anodes and electrolyte ions for copper electrowinning
[J]. Mater. Prot. , 2020 , 53 (8 ): 117
王秀凯 , 陈 胜 , 陈步明 等 铜电积用节能阳极及电解液离子影响的研究现状
[J]. 材料保护 , 2020 , 53 (8 ): 117
[5]
Zhuang S W Wu B Duan N et al Research progress in anodes for zinc electrowinning
[J]. Acta Mater. Compos. Sin. , 2021 , 38 : 1313
庄思伟 , 吴 冰 , 段 宁 等 锌电沉积阳极的研究进展
[J]. 复合材料学报 , 2021 , 38 : 1313
[6]
Xiong Y L Wang Y F Roselle G et al Lead/lead-alloy as a corrosion-resistant outer layer packaging material for high level nuclear waste disposal
[J]. Nucl. Eng. Des. , 2021 , 380 : 111294
DOI
URL
[本文引用: 1]
[7]
Ahmed T Jiang H X Li W et al Solidification of Pb-Al alloys under the influence of electric current pulses
[J]. Acta Metall. Sin. (Engl. Lett.) , 2018 , 31 : 842
DOI
URL
[本文引用: 1]
[8]
Li S R Lead and Lead Alloy [M]. Changsha : Central South University Press , 1996 : 262
[本文引用: 1]
李松瑞 铅及铅合金 [M]. 长沙 : 中南大学出版社 , 1996 : 262
[本文引用: 1]
[9]
Cole J F Goodwin F E The development and potential applications of a Pb-Al alloy
[J]. JOM , 1990 , 42 : 41
DOI
URL
[10]
Cao Y D Preparation and performance of a novel lead-based alloy anode for zinc electrowinning
[D]. Kunming : Kunming University of Science and Technology , 2009
[本文引用: 1]
曹远栋 锌电积用新型铅基合金阳极的制备及性能研究
[D]. 昆明 : 昆明理工大学 , 2009
[本文引用: 1]
[11]
Li W Sun Q Jiang H X et al Solidification of Al-Bi alloy and influence of microalloying element Sn
[J]. Acta Metall. Sin. , 2019 , 55 : 831
[本文引用: 1]
黎 旺 , 孙 倩 , 江鸿翔 等 Al-Bi合金凝固过程及微合金化元素Sn的影响
[J]. 金属学报 , 2019 , 55 : 831
[本文引用: 1]
[12]
Zhao J Z Ahmed T Jiang H X et al Solidification of immiscible alloys: A review
[J]. Acta Metall. Sin. (Engl. Lett.) , 2017 , 30 : 1
DOI
URL
[13]
Deng C K Jiang H X Zhao J Z et al Study on the solidification of Ag-Ni monotectic alloy
[J]. Acta Metall. Sin. , 2020 , 56 : 212
[本文引用: 2]
邓聪坤 , 江鸿翔 , 赵九洲 等 Ag-Ni偏晶合金凝固过程研究
[J]. 金属学报 , 2020 , 56 : 212
DOI
[本文引用: 2]
对Ag-Ni偏晶合金开展了快速/亚快速凝固实验,获得了富Ni相粒子均匀弥散分布于Ag基体的合金样品,Ag-Ni合金显微硬度随着合金Ni含量增加和试样凝固过程冷却速率升高而增大,当Ag-4.0%Ni合金液-液相变开始阶段熔体冷却速率达1800 K/s时,其显微硬度接近粉末冶金生产的Ag-10.0%Ni片状电触头的硬度。建立了描述Ag-Ni合金凝固组织演变的动力学模型,模拟计算了Ag-Ni合金凝固组织形成过程,分析讨论了合金成分和试样直径(冷却速率)对Ag-Ni合金凝固组织形成过程的影响。结果表明:富Ni相液滴/粒子形核阶段熔体的冷却速率对合金凝固组织弥散度具有决定性影响;合金的Ni含量越高、试样冷却速率越低,凝固组织中富Ni相粒子平均尺寸越大;Ag-Ni合金熔体冷却凝固时,富Ni相液滴/粒子的尺寸主要受形核和长大控制,Ostwald粗化作用很弱。
[14]
Gránásy L Ratke L Homogeneous nucleation within the liquid miscibility gap of Zn-Pb alloys
[J]. Scr. Metall. Mater. , 1993 , 28 : 1329
DOI
URL
[本文引用: 1]
[15]
Ratke L Diefenbach S Liquid immiscible alloys
[J]. Mater. Sci. Eng. , 1995 , R15 : 263
[本文引用: 1]
[16]
Li H L Study of the mechanism of the microstructure evolution in directionally solidified monotectic alloys
[D]. Shenyang : Institute of Metal Research, Chinese Academy of Sciences , 2009
[本文引用: 1]
李海丽 偏晶合金定向凝固过程中组织演变机理研究
[D]. 沈阳 : 中国科学院金属研究所 , 2009
[本文引用: 1]
[17]
Zhao J Ratke L Repeated nucleation of minority phase droplets induced by drop motion
[J]. Scr. Mater. , 1998 , 39 : 181
DOI
URL
[本文引用: 1]
[18]
Jiang H X Zhao J Z Effect mechanism of a direct current on the solidification of immiscible alloys
[J]. Chin. Phys. Lett. , 2012 , 29 : 088104
[本文引用: 1]
[19]
Zhao J Z Ratke L Jia J et al Modeling and simulation of the microstructure evolution during a cooling of immiscible alloys in the miscibility gap
[J]. J. Mater. Sci. Technol. , 2002 , 18 : 197
[本文引用: 1]
[20]
Shangguan D Ahuja S Stefanescu D M An analytical model for the interaction between an insoluble particle and an advancing solid/liquid interface
[J]. Metall . Trans., 1992 , 23A : 669
[本文引用: 1]
[21]
Li H L Zhao J Z Convective effect on the microstructure evolution during a liquid-liquid decomposition
[J]. Appl. Phys. Lett. , 2008 , 92 : 241902
DOI
URL
[本文引用: 1]
[22]
Einstein A A new determination of molecular dimensions
[J]. Ann. Phys. , 1906 , 324 : 289
DOI
URL
[本文引用: 1]
[23]
Yu S K Sommer F Predel B Isopiestic measurements and assessment of the Al-Pb System
[J]. Z. Metallkd. , 1996 , 87 : 574
[本文引用: 1]
[24]
Takamichi I Roderick I L G translated by Xian A P Wang L W The Physical Properties of Liquid Metals [M]. Beijing : Science Press , 2006 : 256
[本文引用: 3]
Takamichi I, Roderick I L G著, 冼爱平, 王连文译. 液态金属的物理性能 [M]. 北京 : 科学出版社 , 2006 : 256
[本文引用: 3]
[25]
Li H L Zhao J Z Zhang Q X et al Microstructure formation in a directionally solidified immiscible alloy
[J]. Metall. Mater. Trans. , 2008 , 39A : 3308
[本文引用: 1]
铅酸电池泡沫铅板栅材料的研究进展
1
2006
... Pb是湿法冶金阳极、蓄电池板栅、防辐射等的重要材料[1 ~6 ] .为了满足强度、耐蚀、导电等性能需求,工业上常用铅基合金,如湿法冶金中使用Pb-(0.8~1.2)Ag (质量分数,%,下同)合金作为阳极材料,蓄电池中使用Pb-Sb和Pb-Ca合金作为板栅材料等.Al密度低、导电率高、力学性能好,在改善铅合金性能方面具有很大的潜力[7 ] .当以纳/微米粒子的形式均匀弥散地分布于Pb基体中时,Al可以提高铅基合金的导电性及强度,改善铅基合金的使用性能.研究结果[8 ~10 ] 已证明:原位Al粒子Pb-Al合金是一种潜在的高性能、低成本的湿法冶金阳极或铅蓄电池板栅材料;添加0.01~0.02的Al即可显著提高Pb-Ca合金耐蚀性;添加0.11的Al即能显著提高锌湿法冶金阳极合金Pb-Ag的强度和耐蚀性等. ...
铅酸电池泡沫铅板栅材料的研究进展
1
2006
... Pb是湿法冶金阳极、蓄电池板栅、防辐射等的重要材料[1 ~6 ] .为了满足强度、耐蚀、导电等性能需求,工业上常用铅基合金,如湿法冶金中使用Pb-(0.8~1.2)Ag (质量分数,%,下同)合金作为阳极材料,蓄电池中使用Pb-Sb和Pb-Ca合金作为板栅材料等.Al密度低、导电率高、力学性能好,在改善铅合金性能方面具有很大的潜力[7 ] .当以纳/微米粒子的形式均匀弥散地分布于Pb基体中时,Al可以提高铅基合金的导电性及强度,改善铅基合金的使用性能.研究结果[8 ~10 ] 已证明:原位Al粒子Pb-Al合金是一种潜在的高性能、低成本的湿法冶金阳极或铅蓄电池板栅材料;添加0.01~0.02的Al即可显著提高Pb-Ca合金耐蚀性;添加0.11的Al即能显著提高锌湿法冶金阳极合金Pb-Ag的强度和耐蚀性等. ...
The influences of silver and zinc addition on the electrochemical performances of the Pb-Ca-Sn grids for lead acid batteries
0
2018
Investigation of Pb-Sr and Pb-Ca binary alloys as grids for lead-acid batteries
0
2019
铜电积用节能阳极及电解液离子影响的研究现状
0
2020
铜电积用节能阳极及电解液离子影响的研究现状
0
2020
Lead/lead-alloy as a corrosion-resistant outer layer packaging material for high level nuclear waste disposal
1
2021
... Pb是湿法冶金阳极、蓄电池板栅、防辐射等的重要材料[1 ~6 ] .为了满足强度、耐蚀、导电等性能需求,工业上常用铅基合金,如湿法冶金中使用Pb-(0.8~1.2)Ag (质量分数,%,下同)合金作为阳极材料,蓄电池中使用Pb-Sb和Pb-Ca合金作为板栅材料等.Al密度低、导电率高、力学性能好,在改善铅合金性能方面具有很大的潜力[7 ] .当以纳/微米粒子的形式均匀弥散地分布于Pb基体中时,Al可以提高铅基合金的导电性及强度,改善铅基合金的使用性能.研究结果[8 ~10 ] 已证明:原位Al粒子Pb-Al合金是一种潜在的高性能、低成本的湿法冶金阳极或铅蓄电池板栅材料;添加0.01~0.02的Al即可显著提高Pb-Ca合金耐蚀性;添加0.11的Al即能显著提高锌湿法冶金阳极合金Pb-Ag的强度和耐蚀性等. ...
Solidification of Pb-Al alloys under the influence of electric current pulses
1
2018
... Pb是湿法冶金阳极、蓄电池板栅、防辐射等的重要材料[1 ~6 ] .为了满足强度、耐蚀、导电等性能需求,工业上常用铅基合金,如湿法冶金中使用Pb-(0.8~1.2)Ag (质量分数,%,下同)合金作为阳极材料,蓄电池中使用Pb-Sb和Pb-Ca合金作为板栅材料等.Al密度低、导电率高、力学性能好,在改善铅合金性能方面具有很大的潜力[7 ] .当以纳/微米粒子的形式均匀弥散地分布于Pb基体中时,Al可以提高铅基合金的导电性及强度,改善铅基合金的使用性能.研究结果[8 ~10 ] 已证明:原位Al粒子Pb-Al合金是一种潜在的高性能、低成本的湿法冶金阳极或铅蓄电池板栅材料;添加0.01~0.02的Al即可显著提高Pb-Ca合金耐蚀性;添加0.11的Al即能显著提高锌湿法冶金阳极合金Pb-Ag的强度和耐蚀性等. ...
1
1996
... Pb是湿法冶金阳极、蓄电池板栅、防辐射等的重要材料[1 ~6 ] .为了满足强度、耐蚀、导电等性能需求,工业上常用铅基合金,如湿法冶金中使用Pb-(0.8~1.2)Ag (质量分数,%,下同)合金作为阳极材料,蓄电池中使用Pb-Sb和Pb-Ca合金作为板栅材料等.Al密度低、导电率高、力学性能好,在改善铅合金性能方面具有很大的潜力[7 ] .当以纳/微米粒子的形式均匀弥散地分布于Pb基体中时,Al可以提高铅基合金的导电性及强度,改善铅基合金的使用性能.研究结果[8 ~10 ] 已证明:原位Al粒子Pb-Al合金是一种潜在的高性能、低成本的湿法冶金阳极或铅蓄电池板栅材料;添加0.01~0.02的Al即可显著提高Pb-Ca合金耐蚀性;添加0.11的Al即能显著提高锌湿法冶金阳极合金Pb-Ag的强度和耐蚀性等. ...
1
1996
... Pb是湿法冶金阳极、蓄电池板栅、防辐射等的重要材料[1 ~6 ] .为了满足强度、耐蚀、导电等性能需求,工业上常用铅基合金,如湿法冶金中使用Pb-(0.8~1.2)Ag (质量分数,%,下同)合金作为阳极材料,蓄电池中使用Pb-Sb和Pb-Ca合金作为板栅材料等.Al密度低、导电率高、力学性能好,在改善铅合金性能方面具有很大的潜力[7 ] .当以纳/微米粒子的形式均匀弥散地分布于Pb基体中时,Al可以提高铅基合金的导电性及强度,改善铅基合金的使用性能.研究结果[8 ~10 ] 已证明:原位Al粒子Pb-Al合金是一种潜在的高性能、低成本的湿法冶金阳极或铅蓄电池板栅材料;添加0.01~0.02的Al即可显著提高Pb-Ca合金耐蚀性;添加0.11的Al即能显著提高锌湿法冶金阳极合金Pb-Ag的强度和耐蚀性等. ...
The development and potential applications of a Pb-Al alloy
0
1990
锌电积用新型铅基合金阳极的制备及性能研究
1
2009
... Pb是湿法冶金阳极、蓄电池板栅、防辐射等的重要材料[1 ~6 ] .为了满足强度、耐蚀、导电等性能需求,工业上常用铅基合金,如湿法冶金中使用Pb-(0.8~1.2)Ag (质量分数,%,下同)合金作为阳极材料,蓄电池中使用Pb-Sb和Pb-Ca合金作为板栅材料等.Al密度低、导电率高、力学性能好,在改善铅合金性能方面具有很大的潜力[7 ] .当以纳/微米粒子的形式均匀弥散地分布于Pb基体中时,Al可以提高铅基合金的导电性及强度,改善铅基合金的使用性能.研究结果[8 ~10 ] 已证明:原位Al粒子Pb-Al合金是一种潜在的高性能、低成本的湿法冶金阳极或铅蓄电池板栅材料;添加0.01~0.02的Al即可显著提高Pb-Ca合金耐蚀性;添加0.11的Al即能显著提高锌湿法冶金阳极合金Pb-Ag的强度和耐蚀性等. ...
锌电积用新型铅基合金阳极的制备及性能研究
1
2009
... Pb是湿法冶金阳极、蓄电池板栅、防辐射等的重要材料[1 ~6 ] .为了满足强度、耐蚀、导电等性能需求,工业上常用铅基合金,如湿法冶金中使用Pb-(0.8~1.2)Ag (质量分数,%,下同)合金作为阳极材料,蓄电池中使用Pb-Sb和Pb-Ca合金作为板栅材料等.Al密度低、导电率高、力学性能好,在改善铅合金性能方面具有很大的潜力[7 ] .当以纳/微米粒子的形式均匀弥散地分布于Pb基体中时,Al可以提高铅基合金的导电性及强度,改善铅基合金的使用性能.研究结果[8 ~10 ] 已证明:原位Al粒子Pb-Al合金是一种潜在的高性能、低成本的湿法冶金阳极或铅蓄电池板栅材料;添加0.01~0.02的Al即可显著提高Pb-Ca合金耐蚀性;添加0.11的Al即能显著提高锌湿法冶金阳极合金Pb-Ag的强度和耐蚀性等. ...
Al-Bi合金凝固过程及微合金化元素Sn的影响
1
2019
... 然而,Pb-Al合金相图中存在范围很宽的液-液两相区及液-固两相区,凝固过程中首先发生液-液/液-固分相——弥散相液滴/粒子自熔体沉淀析出,极易形成弥散相尺寸粗大或相偏析严重的凝固组织[11 ~13 ] ,其凝固过程研究和工业制备受到严重限制.本工作以Pb-0.15Al合金为研究对象,实验研究定向凝固条件下合金的液-固分相与凝固行为,并基于群体动力学方法建立液-固分相合金定向凝固模型,实验与模拟相结合,探索Pb-Al合金液-固分相过程与原位粒子复合凝固组织的形成规律. ...
Al-Bi合金凝固过程及微合金化元素Sn的影响
1
2019
... 然而,Pb-Al合金相图中存在范围很宽的液-液两相区及液-固两相区,凝固过程中首先发生液-液/液-固分相——弥散相液滴/粒子自熔体沉淀析出,极易形成弥散相尺寸粗大或相偏析严重的凝固组织[11 ~13 ] ,其凝固过程研究和工业制备受到严重限制.本工作以Pb-0.15Al合金为研究对象,实验研究定向凝固条件下合金的液-固分相与凝固行为,并基于群体动力学方法建立液-固分相合金定向凝固模型,实验与模拟相结合,探索Pb-Al合金液-固分相过程与原位粒子复合凝固组织的形成规律. ...
Solidification of immiscible alloys: A review
0
2017
Ag-Ni偏晶合金凝固过程研究
2
2020
... 然而,Pb-Al合金相图中存在范围很宽的液-液两相区及液-固两相区,凝固过程中首先发生液-液/液-固分相——弥散相液滴/粒子自熔体沉淀析出,极易形成弥散相尺寸粗大或相偏析严重的凝固组织[11 ~13 ] ,其凝固过程研究和工业制备受到严重限制.本工作以Pb-0.15Al合金为研究对象,实验研究定向凝固条件下合金的液-固分相与凝固行为,并基于群体动力学方法建立液-固分相合金定向凝固模型,实验与模拟相结合,探索Pb-Al合金液-固分相过程与原位粒子复合凝固组织的形成规律. ...
... 合金凝固过程中,热量通过导热、对流进行传输,且受熔体中富Al粒子空间迁移的影响.合金的温度场满足如下方程[13 ] : ...
Ag-Ni偏晶合金凝固过程研究
2
2020
... 然而,Pb-Al合金相图中存在范围很宽的液-液两相区及液-固两相区,凝固过程中首先发生液-液/液-固分相——弥散相液滴/粒子自熔体沉淀析出,极易形成弥散相尺寸粗大或相偏析严重的凝固组织[11 ~13 ] ,其凝固过程研究和工业制备受到严重限制.本工作以Pb-0.15Al合金为研究对象,实验研究定向凝固条件下合金的液-固分相与凝固行为,并基于群体动力学方法建立液-固分相合金定向凝固模型,实验与模拟相结合,探索Pb-Al合金液-固分相过程与原位粒子复合凝固组织的形成规律. ...
... 合金凝固过程中,热量通过导热、对流进行传输,且受熔体中富Al粒子空间迁移的影响.合金的温度场满足如下方程[13 ] : ...
Homogeneous nucleation within the liquid miscibility gap of Zn-Pb alloys
1
1993
... 当均一的Pb-Al合金熔体冷却进入液-固分相温度区间以后,弥散相富Al粒子自熔体中沉淀析出,其形核率(I )可用下式计算[14 ,15 ] : ...
Liquid immiscible alloys
1
1995
... 当均一的Pb-Al合金熔体冷却进入液-固分相温度区间以后,弥散相富Al粒子自熔体中沉淀析出,其形核率(I )可用下式计算[14 ,15 ] : ...
偏晶合金定向凝固过程中组织演变机理研究
1
2009
... 富Al粒子形核后,将在过饱和熔体中扩散长大,长大速率(v R )为[16 ,17 ] : ...
偏晶合金定向凝固过程中组织演变机理研究
1
2009
... 富Al粒子形核后,将在过饱和熔体中扩散长大,长大速率(v R )为[16 ,17 ] : ...
Repeated nucleation of minority phase droplets induced by drop motion
1
1998
... 富Al粒子形核后,将在过饱和熔体中扩散长大,长大速率(v R )为[16 ,17 ] : ...
Effect mechanism of a direct current on the solidification of immiscible alloys
1
2012
... 为了定量地描述合金液-固分相凝固过程中原位富Al粒子复合凝固组织的形成,定义富Al粒子的半径分布函数 f ( r , z , R , t ) f ( r , z , R , t ) d R t ( r , z ) R R + d R f [18 ,19 ] : ...
Modeling and simulation of the microstructure evolution during a cooling of immiscible alloys in the miscibility gap
1
2002
... 为了定量地描述合金液-固分相凝固过程中原位富Al粒子复合凝固组织的形成,定义富Al粒子的半径分布函数 f ( r , z , R , t ) f ( r , z , R , t ) d R t ( r , z ) R R + d R f [18 ,19 ] : ...
An analytical model for the interaction between an insoluble particle and an advancing solid/liquid interface
1
1992
... 式中, u S 2 g ( ρ β - ρ m ) R 2 / ( 9 η m ) g ρ β ρ m η m 为基体熔体黏度),为富Al粒子的Stokes运动速度[20 ] ; V V 0 + V c V 0 V c V 0 V 0 . 为了便于讨论,将以上速度在rOz 坐标系(图1 b,O 为稳态凝固条件下凝固界面的最低点)中分解: u S u S z j V V r i + V z j V 0 V 0 z j V c V c r i + V c z j i j r 、z 方向的单位向量; u S z V r V z V 0 z V c r V c z r 或z 方向的分量,其正负表示方向). ...
Convective effect on the microstructure evolution during a liquid-liquid decomposition
1
2008
... V [21 ] : ...
A new determination of molecular dimensions
1
1906
... 式中,ρ 为合金熔体(基体熔体与富Al粒子的两相混合物)的密度; p η )采用Einstein黏度公式计算[22 ] :η = η m (1 + 2.5ϕ ) (其中,ϕ 为合金熔体内富Al粒子的体积分数). ...
Isopiestic measurements and assessment of the Al-Pb System
1
1996
... 采用有限体积法将以上流场、温度场、浓度场和富Al粒子半径分布函数控制方程离散,并与合金相图[23 ] 进行耦合求解,即可模拟定向凝固条件下液-固分相Pb-Al合金凝固组织形成过程. ...
3
2006
... 模拟计算中使用的参数见表1 [24 ] ,用拟合法确定 h 1 、 h 2 σ σ ) ,选取不同的 h 1 、 h 2 h 1 h 2 2 ·K),进而,使用该有效对流换热系数模拟计算其他凝固速率下合金熔体中心轴线处的温度分布曲线,通过检查实测值与计算结果之间的符合程度(图2 ),进一步验证拟合所得参数值的正确性;然后,基于上述已知参数,选取不同的σ 模拟计算4 mm/s凝固速率时合金凝固组织中富Al粒子的平均半径,通过检查实测值与计算结果之间的符合程度,确定σ = 0.135 J/m2 ,进而,使用该σ 模拟计算其他凝固速率下合金凝固组织中富Al粒子的平均半径,通过检查实测值与计算结果之间的符合程度(图5 ),进一步验证所取σ 的正确性. ...
... Pb-Al体系的热物性参数[24 ] ...
... Thermophysical property parameters of Pb-Al system[24 ] ...
3
2006
... 模拟计算中使用的参数见表1 [24 ] ,用拟合法确定 h 1 、 h 2 σ σ ) ,选取不同的 h 1 、 h 2 h 1 h 2 2 ·K),进而,使用该有效对流换热系数模拟计算其他凝固速率下合金熔体中心轴线处的温度分布曲线,通过检查实测值与计算结果之间的符合程度(图2 ),进一步验证拟合所得参数值的正确性;然后,基于上述已知参数,选取不同的σ 模拟计算4 mm/s凝固速率时合金凝固组织中富Al粒子的平均半径,通过检查实测值与计算结果之间的符合程度,确定σ = 0.135 J/m2 ,进而,使用该σ 模拟计算其他凝固速率下合金凝固组织中富Al粒子的平均半径,通过检查实测值与计算结果之间的符合程度(图5 ),进一步验证所取σ 的正确性. ...
... Pb-Al体系的热物性参数[24 ] ...
... Thermophysical property parameters of Pb-Al system[24 ] ...
Microstructure formation in a directionally solidified immiscible alloy
1
2008
... Pb-Al液-固分相合金形成弥散型凝固组织的前提条件是合金的定向凝固过程可以实现稳态凝固,这要求凝固界面前沿合金熔体内所有富Al粒子(任意尺寸、任意位置)始终向着凝固界面迁移[25 ] ,即满足: ( V 0 z + V c z + u S z ) ∀ R , r , z u S z m a x V c z m a x V 0 z 图15 给出了Pb-0.15Al合金定向凝固过程中 u S z m a x V c z m a x u S z m a x V c z m a x V 0 z V 0 的变化.可见,当富Al粒子不发生Stokes运动且熔体内无对流(u S z m a x V c z m a x V 0 z 图15 中黑色线);当熔体内弥散相粒子Stokes运动方向与凝固方向相同(u S z m a x 图15 中红色线)或存在与凝固方向相同的熔体对流(V c z m a x 图15 中绿色线)时,为了使合金实现稳态凝固, V 0 V 0 V 0 z u S z m a x V c z m a x V 0 c 图15 中蓝色线,临界凝固速率 V 0 c