目前针对非调质钢腐蚀行为的研究大多采用盐雾腐蚀、溶液浸泡等方法,目的在于均匀腐蚀性能。但任何均匀腐蚀都并非完全均匀,金属表面总会同时存在着阴极区和阳极区,尤其是在初期阶段,不同的夹杂物类型、夹杂物形态都有可能对腐蚀产生显著影响。而非调质钢由于整体耐蚀性较弱,其夹杂物对腐蚀行为的影响采用均匀腐蚀的评价方法难以进行揭示。
1 实验方法
实验用材料包括C70S6非调质钢和在此基础上掺Ca、Mg的样品(样品简记为C70S6-CaMg),其中掺杂的Ca、Mg元素含量均为10-6量级。C70S6的化学成分(质量分数,%)为:Cr 0.1,Ni 0.06,Mo 0.002,C 0.72,Mn 0.57,Si 0.21,P 0.006,S 0.062,V 0.0014,Al 0.005,W 0.036,Fe余量。除此之外,在探索pH测试条件时还用到了纯Fe和409L不锈钢,其中409L不锈钢的化学成分(质量分数,%)为:Cr 11.45,Ni 0.12,C 0.010,Mn 0.24,Si 0.42,P 0.015,S 0.008,Cu 0.02,Ti 0.2,N 0.008,Fe余量。
实验样品通过线切割加工成为12 mm × 12 mm × 1 mm的片状样品,从样品的非工作面引出导线,用环氧树脂封装样品,并暴露测试面积为1 cm2。待工作电极封好之后, 用水磨砂纸逐级打磨工作面,依次用蒸馏水、乙醇、丙酮清洗,吹干。
利用CHI660E型电化学工作站对上述材料进行极化曲线测试,采用标准的三电极体系,辅助电极为Pt电极,参比电极为饱和甘汞电极(SCE),工作电极为样品。首先将工作电极浸入添加不同浓度NaOH和NaCl (控制值pH和Cl-浓度[Cl-])的0.1 mol/L浓度Na2SO4溶液中,先进行-900 mV的恒电位阴极极化120 s,而后维持在开路状态下稳定900 s,最后施加动电位从开路电位(OCP)处开始以一定的扫描速率向阳极方向扫描,直到稳态点蚀或者过钝化发生,电流上升达到一定数值,停止实验。
采用pH调制方法,考察纯Fe在不同pH值下是否如409L不锈钢一样,随着pH值的升高,改善其表面性能,出现钝化区。溶液的pH值由pH计实测得出,每次实验前均对pH计进行校准。
对C70S6和C70S6-CaMg样品进行打磨并用2.5 μm金刚石悬浊液抛光。采用上海立光4XG金相显微镜(OM)观察样品的点蚀形貌,并采用Gemini 300扫描电镜(SEM)对测试前后的C70S6系列样品夹杂物进行尺寸统计及能谱(EDS)表征。
2 实验结果与讨论
2.1 掺Ca、Mg对夹杂物尺寸的影响
表1 C70S6非调质钢中夹杂物类型、数量及尺寸分布
Table 1
| Inclusion type | Number | 0-2 μm | 2-5 μm | 5-10 μm | > 10 μm |
|---|---|---|---|---|---|
| MnS | 4750 | 2487 | 2064 | 198 | 1 |
| MgO-MnS | 12 | 7 | 3 | 2 | 0 |
| CaO-MnS | 18 | 10 | 8 | 0 | 0 |
| Al2O3-MnS | 105 | 63 | 35 | 7 | 0 |
| Spinel-MnS | 77 | 34 | 40 | 3 | 0 |
| Aluminate-MnS | 157 | 68 | 76 | 13 | 0 |
| Others | 23 | 11 | 8 | 4 | 0 |
| Total | 5142 | 2680 | 2234 | 227 | 1 |
表2 C70S6-CaMg非调质钢中夹杂物类型、数量及尺寸分布
Table 2
| Inclusion type | Number | 0-2 μm | 2-5 μm | 5-10 μm | > 10 μm |
|---|---|---|---|---|---|
| MnS | 6774 | 4949 | 1777 | 48 | 0 |
| MgO-MnS | 62 | 39 | 22 | 1 | 0 |
| CaO-MnS | 3 | 2 | 1 | 0 | 0 |
| Al2O3-MnS | 207 | 142 | 62 | 3 | 0 |
| Spinel-MnS | 230 | 161 | 67 | 2 | 0 |
| Aluminate-MnS | 445 | 299 | 136 | 10 | 0 |
| Others | 49 | 31 | 17 | 1 | 0 |
| Total | 7770 | 5623 | 2082 | 65 | 0 |
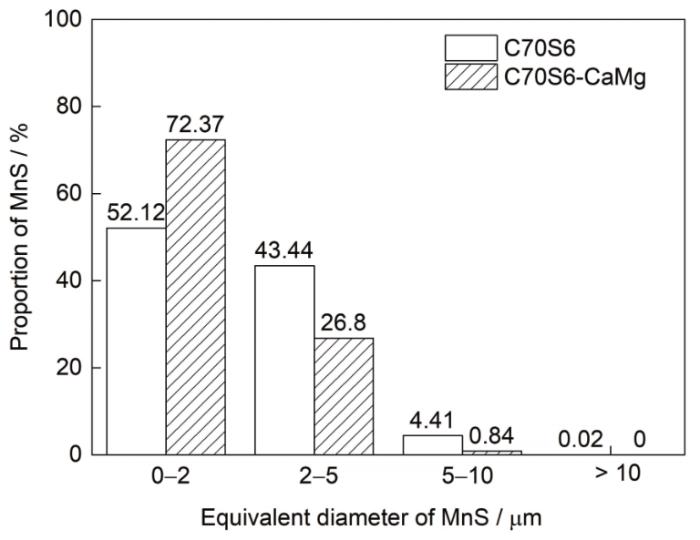
图1
图1
C70S6及C70S6-CaMg非调质钢中MnS夹杂物等效直径分布图
Fig.1
Equivalent diameter distribution of MnS inclusions in C70S6 and C70S6-CaMg non-quenched and tempered steel
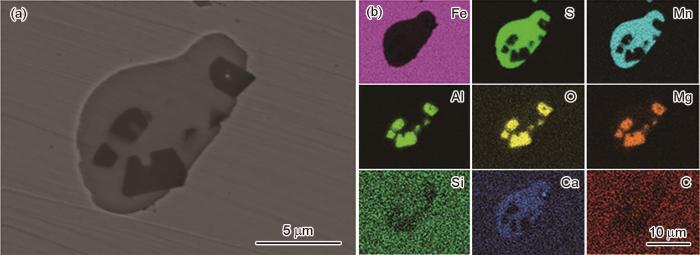
2.2 C70S6夹杂物的形貌表征
图2
图2
C70S6夹杂形貌夹杂物的微观形貌及EDS元素分布图
Fig.2
Inclusion morphology (a) and element distribution maps (b) of C70S6
图3
图3
C70S6-CaMg夹杂形貌及EDS元素分布图
Fig.3
Inclusion morphology (a) and element distribution maps (b) of C70S6-CaMg
2.3 C70S6系列非调质钢极化曲线测试
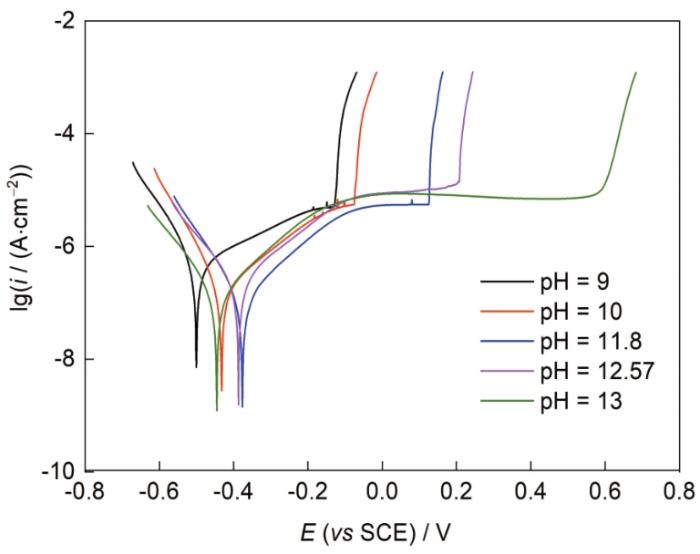
可以看出,随着pH值的升高,点蚀电位逐渐向正方向移动,钝化区持续变宽,直到pH值为13时,样品一直到发生放氧反应也未出现点蚀。这表明,溶液碱性提高使不锈钢获得的钝性,能够定性地对应于合金化程度提高带来的钝性。
图4
图4
不同碱性条件下409L不锈钢的极化曲线
Fig.4
Polarization curves of 409L stainless steel under different alkaline conditions
为减少材料本身的干扰因素,采用纯Fe作为条件探索样品进行测试,图5是不同pH值下纯Fe的极化曲线。可以看出,在pH = 11.2~11.8之间时出现了明显的活化钝化转变,当pH > 11.8时,非钝性的纯Fe也出现了钝化区,但样品并未发生点蚀,电流上升值集中在放氧电位0.6 V左右。在此基础上本实验于pH = 12.6时添加了不同浓度的Cl-。
图5
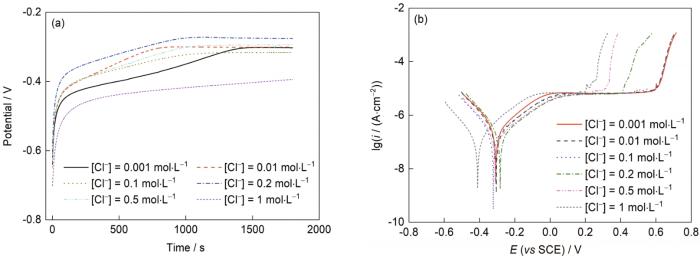
图6是纯Fe在pH = 12.6时分别添加0.001、0.01、0.1、0.2、0.5和1 mol/L Cl-时的极化曲线结果。实验表明,在该pH值下0.01 mol/L及以下的Cl-浓度对试样仍然几乎没有影响,但在0.1 mol/L及以上的Cl-浓度溶液环境中进一步提高Cl-浓度后,发现在溶液pH = 12.6、[Cl-] ≥ 0.1 mol/L时,随着Cl-浓度的升高,电流突升点逐渐降低。实验结果表明通过调制合适的溶液环境可以使弱钝性铁合金获得与不锈钢相似的钝性。
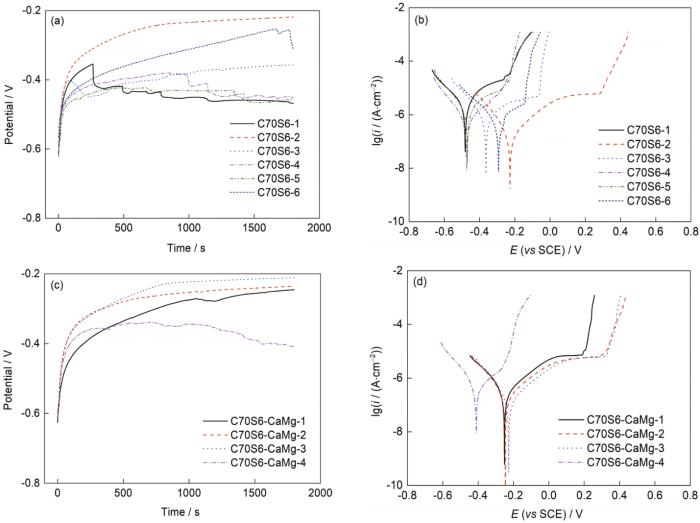
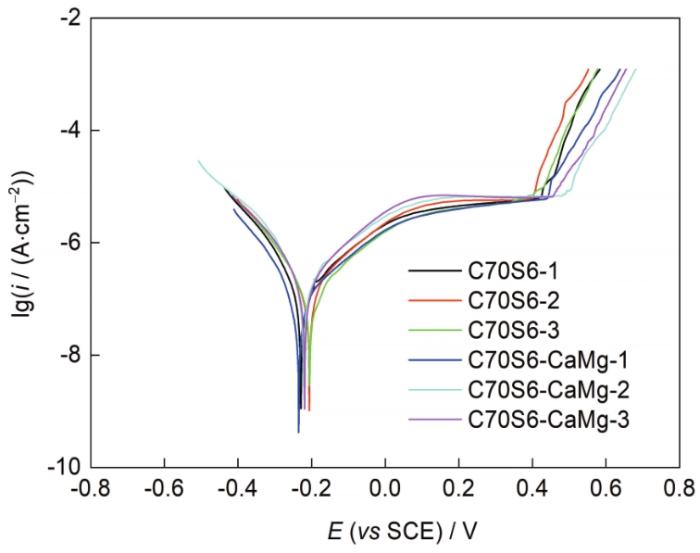
对C70S6和C70S6-CaMg样品进行动电位极化曲线测试。在纯Fe溶液条件探索的前提下考虑到非调质钢相较于纯Fe有一定的钝性,因此实验选择pH = 12.6、[Cl-] = 1 mol/L的条件,以便能有较大的调整空间。实验结果如图7所示。图7表明,相较于纯Fe,掺杂一定量钝性元素的非调质钢显然有更好的耐点蚀性能。掺杂样品和未掺杂样品极化曲线基本重合,电流突升电位接近放氧电位,因此考虑进一步采用更加苛刻的条件进行研究。实验选取pH = 12、[Cl-] = 0~1 mol/L下C70S6-CaMg的OCP和极化曲线,如图8所示。结果表明,当[Cl-] > 0.1 mol/L时难以钝化完全,因此选取pH = 12、[Cl-] = 0.1 mol/L时的临界条件下对2者进行多次实验,结果如图9所示,其极化曲线测试后的蚀坑形貌如图10所示。
图6
图6
pH = 12.6时不同Cl-浓度[Cl-]下纯Fe的开路电位(OCP)和极化曲线
Fig.6
Open circuit potential (OCP) (a) and polarization (b) curves of pure iron with different Cl- concentration [Cl-] at pH = 12.6
图7
图7
pH = 12.6、[Cl-] = 1 mol/L条件下Fe、C70S6和C70S6-CaMg的OCP和极化曲线
Fig.7
OCP (a) and polarization (b) curves of Fe, C70S6, and C70S6-CaMg at pH = 12.6 and [Cl-] = 1 mol/L
图8
图8
pH = 12、不同Cl-浓度下C70S6-CaMg的OCP和极化曲线
Fig.8
OCP (a) and polarization (b) curves of C70S6-CaMg at different [Cl-] and pH = 12
图9
图9
pH = 12、[Cl-] = 0.1 mol/L条件下C70S6及C70S6-CaMg样品的OCP和极化曲线
Fig.9
OCP (a, c) and polarization (b, d) curves of C70S6 (a, b) and C70S6-CaMg (c, d) at pH = 12 and [Cl-] = 0.1 mol/L
图10
图10
C70S6-CaMg和C70S6极化曲线测试后的蚀坑形貌
Fig.10
Etch pit morphologies of C70S6-CaMg (a) and C70S6 (b) after polarization curve test
根据图9中数据所示,在pH = 12、[Cl-] = 0.1 mol/L条件下, C70S6-CaMg样品的稳定性和钝化性能明显高于C70S6样品,且在该条件下2者的点蚀行为均不稳定,而在表观形貌上C70S6-CaMg样品的蚀坑以较大的坑状形貌为主,C70S6样品表面观察蚀坑则是以多点弥散为主。
图11
图11
pH = 12.3、[Cl-] = 0.1 mol/L条件下C70S6和C70S6-CaMg的极化曲线
Fig.11
Polarization curves of C70S6 and C70S6-CaMg at pH = 12.3 and [Cl-] = 0.1 mol/L
上述实验结果表明,在该条件下C70S6-CaMg的点蚀电位明显高于C70S6,而在蚀坑形貌上,C70S6-CaMg的蚀坑以大块单个点蚀为主,而C70S6样品在主蚀坑周围存在大量小型蚀坑。
2.4 C70S6系列非调质钢点蚀形貌表征
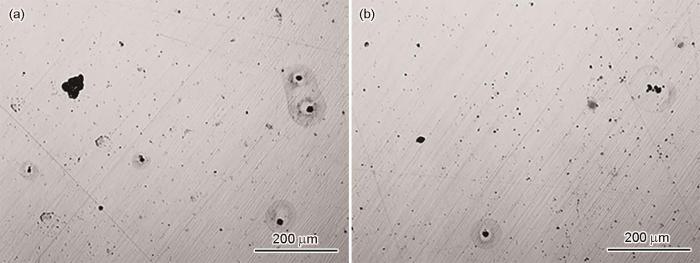
C70S6和C70S6-CaMg样品通过在600 mV下恒电位5 s,激发点蚀活性位点,结果如图13所示。经统计,在C70S6中,点蚀密度为185 mm-2,点蚀坑的平均尺寸约为23.1 μm,最大尺寸约为64 μm;C70S6-CaMg中,点蚀密度为406 mm-2,点蚀坑的平均尺寸约为4.7 μm,最大尺寸约为23.0 μm。整体来看,添加Ca、Mg后,点蚀密度提高2.2倍,点蚀坑更加弥散分布,但点蚀坑的平均尺寸和最大尺寸大幅度减小。
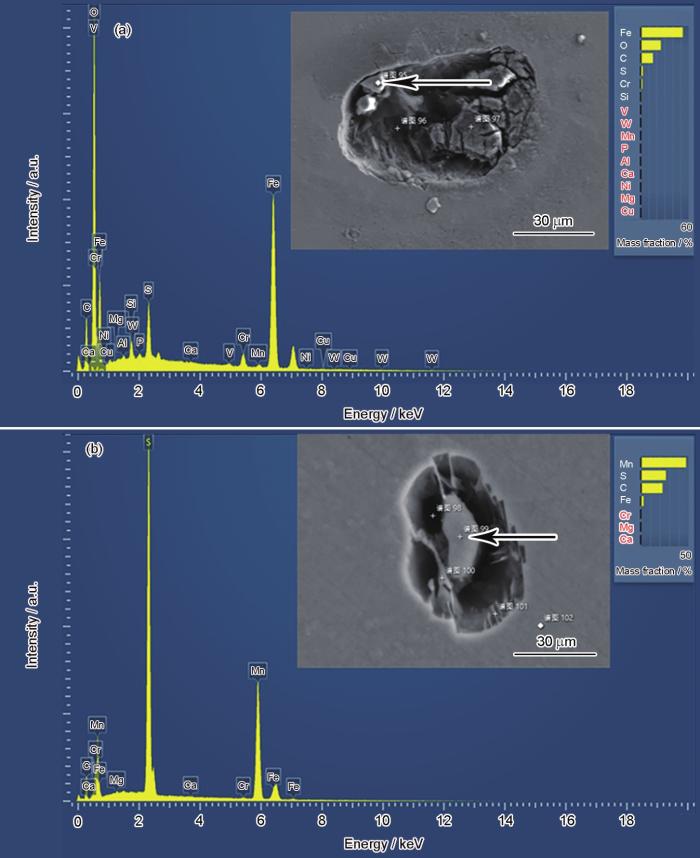
通过对2者蚀坑的EDS表征发现,样品萌生的较大点蚀坑直径在100 μm左右,而周围分布的小型蚀坑直径在5~10 μm范围。整个蚀坑周围以主蚀坑为中心出现圆形氧化物覆盖区域,且MnS夹杂导致的大小蚀坑均在此范围内出现,推测为主蚀坑发生点蚀时,内部酸性溶液不断扩散,致使周围溶液酸化,酸化后的溶液接触到的基体在夹杂物处萌生了小型蚀坑。
图12
图12
pH = 12.3、[Cl-] = 0.1 mol/L时,C70S6-CaMg及C70S6的蚀坑形貌
Fig.12
Pit morphologies of C70S6-CaMg (a) and C70S6 (b) at pH = 12.3 and [Cl-] = 0.1 mol/L
图13
图13
C70S6和C70S6-CaMg样品经恒电位极化后的OM像
Fig.13
OM images of C70S6 (a) and C70S6-CaMg (b) samples after potentiostatic polarization
图14
图14
C70S6主蚀坑形貌及EDS元素分布图
Fig.14
Main pit morphology (a) and element distribution maps (b) of C70S6
图15
图15
C70S6夹杂蚀坑形貌及EDS元素分布图
Fig.15
Pit morphology (a) and element distribution maps (b) of C70S6
图16
图16
C70S6主蚀坑成分及夹杂蚀坑成分
Fig.16
Main pit (a) and inclusion pit (b) (insets) compositions of C70S6
图17
图17
C70S6-CaMg夹杂蚀坑形貌及EDS元素分布图
Fig.17
Pit morphology (a) and element distribution maps (b) of C70S6-CaMg (Red arrows point to the dissolved grooves between the matrix and the MnS;yellow arrows point to the dissolved grooves between sulfides and oxides)
而实验中观察到的掺Ca、Mg样品耐点蚀性能优于不掺Ca、Mg样品的现象,结合文献[9,10,24~28]可知,随着Ca和Mg的加入,由于其与O、S等元素组成的化合物利于成核,使得非调质钢内常见的MnS以MgO和Al2O3为核组成夹杂物,一方面使得夹杂物数量更多,另一方面使得夹杂物总体变小,整体分布更弥散,又由于其形核内部存在不反应的MgO和Al2O3,因此总体上与不掺Ca、Mg样品相比,夹杂物中MnS含量更低,因此更难被侵蚀,而MnS夹杂更大、含量更高的不掺Ca、Mg样品夹杂物更容易发生点蚀产生蚀坑,因此点蚀破裂电位更低,抗点蚀性能更差。而图12中C70S6样品点蚀坑周围的圆环,在图14中显示应该是Fe的氧化物,结合主蚀坑周围大量暴露的MnS夹杂坑,推测应该是主蚀坑点蚀过程中溶液浓差扩散时导致周围溶液酸化,使得蚀坑周围的MnS夹杂物与周围基体纷纷溶解。而掺Ca、Mg样品夹杂物总体反应难度大于未掺Ca、Mg样品,一方面夹杂物更难反应,另一方面也更难提供足够的酸化溶液诱使主蚀坑周围夹杂发生反应。
3 结论
(1) C70S6系列非调质钢内部夹杂主要为MnS,Mg、Al等元素主要以氧化物的状态作为夹杂物内核,Ca、Mn主要以硫化物的形式包裹在外层。
(2) 在合适的溶液条件下(pH = 12.3,[Cl-] = 0.1 mol/L),极化曲线测试反映出C70S6掺Ca、Mg后比C70S6耐点蚀性能更强,结合OM和SEM观察,推测与夹杂有关。
(3) C70S6和C70S6-CaMg样品夹杂物均为包裹结构。C70S6夹杂物中心主要成分为Al2O3,外层为MnS;C70S6-CaMg样品夹杂物中心主要成分为MgO和Al2O3,外层为MnS和CaS。
(4) 由于C70S6-CaMg样品相较于C70S6样品夹杂物中易溶解的MnS含量更少,因此耐点蚀性能更强,更难发生点蚀。
参考文献
Accelerate implementation of green manufacturing of the steel industry to improve level of green development
[J].
加快钢铁“绿色制造”提升绿色发展水平
[J].
Application and prospect of non-quenched tempered steel
[J].
非调质钢的应用及展望
[J].
Application status and prospect of non-quenched and tempered steel in construction machinery
[J].
非调质钢在工程机械上的应用现状及展望
[J].
Application and development of non-quenched and tempered steel for automotive parts
[J].
汽车零部件用非调质钢的应用和发展
[J].
Investigation on non-metallic inclusions in hot forging steels of high cleanliness
[J].
高洁净度汽车用非调质钢中非金属夹杂物研究
[J].
Effect of MnS inclusions distribution on intragranular ferrite formation in medium carbon non-quenched and tempered steel for large-sized crankshaft
[J].
Influence of inclusions on initiation of pitting corrosion and stress corrosion cracking of X70 steel in near-neutral pH environment
[J].
Precipitation and growth of MnS inclusion in an austenitic hot-work die steel during ESR solidification process
[J].
Effect of Mg addition on the evolution of inclusions in Al-Ca deoxidized melts
[J].
Effect of calcium treatment on change behavior of inclusions in sulfur-containing non-quenched and tempered steel
[J].
钙处理对含硫非调质钢中夹杂物演变行为的影响
[J].
Morphology study on inclusion modifications using Mg-Ca treatment in resulfurized special steel
[J].
Improvement of microstructure and properties of non-quenched and tempered steel for hot forging with calcium treatment
[J].
Ca处理对含硫热锻微合金非调质钢组织性能改善的研究
[J].
Study on the corrosion behavior induced by typical inclusions in 304 stainless steel
[D].
304不锈钢中典型夹杂物诱发腐蚀行为研究
[D].
Compositional evolution of oxide inclusions in austenitic stainless steel during continuous casting
[J].
Effects of non-metallic inclusions on the initiation of pitting corrosion in 11% Cr ferritic stainless steel examined by micro-droplet cell
[J].
The effect of different non-metallic inclusions on the machinability of steels
[J].
Influence of inclusions on early corrosion development of ultra-low carbon Bainitic steel in NaCl solution
[J].
Influence of inclusions on initiation of pitting corrosion and stress corrosion cracking of X70 steel in near-neutral pH environment
[J].
Acidic/caustic alternating corrosion on carbon steel pipes in heat exchanger of ethylene plant
[J].
Corrosion resistances of passive films on low-carbon rebar and fine-grained rebar in alkaline media
[J].
普通低碳钢与细晶粒钢钝化膜在碱性介质中的耐蚀性
[J].

应用循环伏安与动电位极化曲线确定普通低碳钢与细晶粒钢在碱性介质(模拟混凝土孔隙液)中的钝化区域. 利用计时电流法在选取的阳极极化电位下使钢筋生成稳定的钝化膜, 并通过电化学阻抗谱、 Mott-Schottky曲线比较了钢筋在不同阳极电位下形成的钝化膜的优劣性; 其次, 循环极化曲线对比分析了在有无Cl<sup>-</sup>存在时普通低碳钢与细晶粒钢钝化膜的耐蚀性. 结果表明, 2种钢筋的公共钝化电位区域为 -0.25-+0.6 V, 在选取的+0.3 V阳极极化电位下2者均能形成更稳定的钝化膜. 在无Cl<sup>-</sup>存在的条件下, 细晶粒钢钝化膜的稳定性与耐蚀性均略优于普通低碳钢; 但有Cl<sup>-</sup>存在时, 细晶粒钢抑制Cl<sup>-</sup>点蚀的能力稍弱于普通低碳钢. 影响细晶粒钢钝化膜耐蚀性的主要原因是晶界数量与微量元素含量.
Effect of sodium thiosulfate on corrosion behavior of Q235 steel in alkaline solution
[J].
硫代硫酸钠对Q235钢碱性腐蚀行为的影响
[J].
Localized corrosion: Passive film breakdown vs. pit growth stability: Part III. A unifying set of principal parameters and criteria for pit stabilization and salt film formation
[J].
Effects of Al-Mg alloy treatment on behavior and size of inclusions in SUH 409L stainless steel
[J].
Effect of Mg addition on carbides in H13 steel during electroslag remelting process
[J].
Formation of MgO-based inclusions during AOD and ladle treatment of Al-killed 2205 duplex stainless steel
[J].
Inclusions modification and improvement of machinability in a non-quenched and tempered steel with Mg treatment
[J].