块体非晶合金在三维空间的原子排列为拓扑无序的玻璃态,而在不超过4~5个原子间距存在1.5 nm范围内化学短程有序或拓扑短程有序区,无位错、晶界、偏析等结晶缺陷,室温下其强度、硬度远高于同成分的晶态金属合金,具有接近于理论极限的断裂强度,并且兼具优异的软磁性能和耐腐蚀性,已成为集众多优点于一身的新型高性能材料[1,2,3]。其中铁基非晶合金复合材料兼具良好的功能特性与结构性能,以其耐腐蚀性能良好、价格低廉得到研究工作者广泛关注[4]。潘杰等[5]采用浸泡法研究了Fe43.7Co7.3Cr14.7Mo12.6C15.5B4.3Y1.9块体非晶合金的腐蚀行为,发现合金在强酸介质中具有优异的耐蚀性,在王水中浸泡236 h的平均腐蚀速率远远小于不锈钢,仅为不锈钢的1/130,合金表面发生均匀腐蚀,钝化膜中Cr元素的富集是其具有强耐蚀能力的主要原因。Fan等[6]研究了Fe41Co7Cr15Mo14C15B6Y2非晶合金在H2SO4溶液中的腐蚀行为,发现合金在1 mol/L 的H2SO4 中的耐蚀性能优于不锈钢(SUS321),且与钛合金(Ti6Al4V)相当。非晶合金表面形成了双层保护膜结构,其内膜由 MoO2、Cr2O3、CoO和FeO组成,外膜由MoO3、Cr(OH)3、Co(OH)2和Fe(OH)3组成。Gostin 等[7]将 (Fe44.3Cr5Co5Mo12.8Mn11.2C15.8B5.9)98.5Y1.5非晶合金与其所对应的晶体材料以及传统钢X210Cr12 (X代表高合金)的耐蚀性能做比较,结果表明,在酸性溶液中非晶合金显示出更高的稳定性,且随着pH值的增加,元素组成的影响更加显著,其中Mo、Mn和Co是降低合金稳定性的关键元素。NaKa等[8]研究了类金属元素对铁基非晶合金耐腐蚀性能的影响,发现在Fe-P-C合金中添加Mo、W、Cr可以显著提高其耐蚀性能。Jayaraj等[9]研究证明,当Cr含量相同时,铁基非晶合金的耐蚀性能优越于SUS316L不锈钢。关于Cr元素对铁基非晶合金耐蚀性能的影响,Pardo等[10]也进行了研究,发现在直流电化学H2SO4环境中,Cr元素的添加有利于钝化膜的形成并提高钝化膜的稳定性,从而降低腐蚀速率;在Fe-C非晶合金中添加Cr和Mo,均可降低非晶合金的腐蚀速率,尤其是当添加一定量的Cr时,Fe-Cr-C合金可在1 mol/L HCL中发生自钝化现象。因此,非晶合金的组元和成分对其耐腐蚀性能有重要影响。
本工作选择价格低廉且具有一定非晶形成能力的新型Fe-15Mn-5Si-14Cr-0.2C (质量分数,%)合金,其微观组织为非晶和奥氏体及铁素体组成的复合结构,研究了试样的组织与力学性能的关系,并研究其在人工海水中的耐腐蚀性能,分析其腐蚀产物,探讨其腐蚀过程和腐蚀机理。
1 实验方法
选用纯度均大于99.9%的Fe、Mn、Cr和C,以及纯度高于99.999%的Si,配置成分为Fe-15Mn-5Si-14Cr-0.2C的合金,总质量60 g。实验选用水冷磁悬浮熔炼炉,在高纯Ar气和水冷条件保护下反复熔炼3次,然后采用铜模负压吸铸法制得直径为2 mm的棒状合金试样。采用EMPYREAN X射线衍射仪(XRD,Cu靶,电压40 kV,平均电流30 mA,扫描角度20°~80°,扫描速率6°/min)和EG450热场发射扫描电镜(SEM)及其附带的电子背散射衍射(EBSD)分析和观察试样成分及组织形貌,利用WDW-100型万能力学试验机(加载速率为0.05 mm/min,试样高径比2∶1)测量试样的室温压缩力学性能。选用三电极体系μAUTOLAB TYPEIII型电化学工作站进行电化学实验,试样为工作电极,参比电极为Ag|AgCl|Cl-,辅助电极为Pt片,测试试样在298 K的人工海水[11] (具体成分为24.53 g/L NaCl+5.20 g/L MgCl2+4.09 g/L Na2SO4+1.16 g/L CaCl2+0.695 g/L KCl+0.201 g/L NaHCO3+0.101 g/L KBr+0.027 g/L H3BO3+0.025 g/L SrCl2+0.003 g/L NaF,采用蒸馏水配置,超声波震荡搅拌均匀后用1 mol/L NaOH将pH值调至8.2)中的腐蚀行为。在人工海水中通电浸泡30 min测量试样的开路电位,待开路电位稳定后,测试其极化曲线和交流阻抗曲线,并由SEM及其附带的能谱(EDS)观察和分析其腐蚀形貌和元素组成,通过背散射(BS)像观察其钝化膜厚度。
2 实验结果与分析
2.1 Fe-15Mn-5Si-14Cr-0.2C试样的微观组织
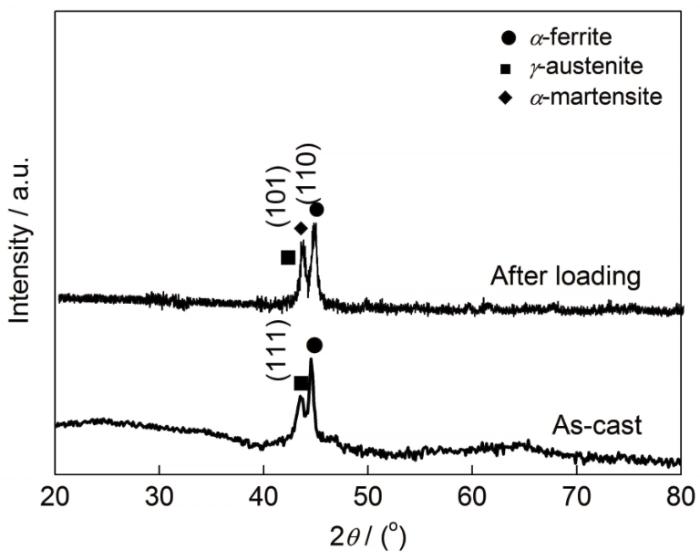
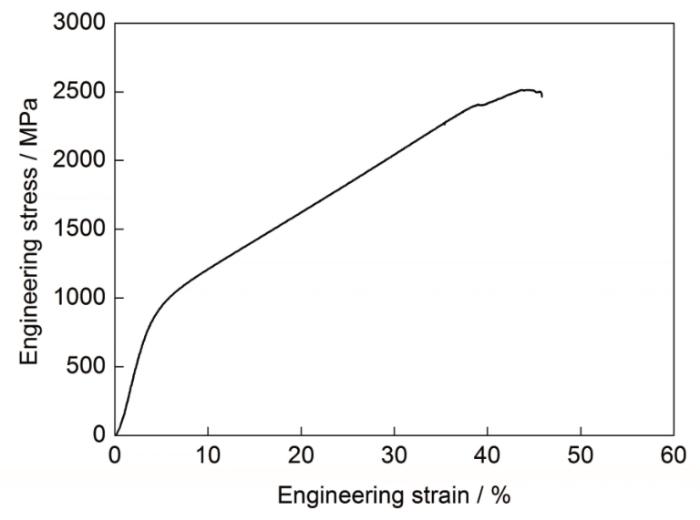
图1为Fe-15Mn-5Si-14Cr-0.2C铸态试样和加载断裂后的XRD谱。可以看出,试样在40°~50°之间均存在明显的非晶漫散峰,且在漫散射峰上有尖锐的晶体衍射峰叠加,因此,试样为非晶相和晶体相(Fe-Cr铁素体相和过冷CFe15.1奥氏体相)组成的复合材料,加入Cr元素可以提高奥氏体强度[12];断裂后生成马氏体相,表明部分奥氏体在加载过程诱发转变为马氏体,相变诱发塑性(transformation induced plasticity,TRIP) 效应显著,使复合材料同时增强增韧。图2是铸态试样的室温工程应力-应变曲线。可见,铸态试样屈服强度、断裂强度和塑性应变分别为978 MPa、2645 MPa和35.8%,并表现为强烈的加工硬化行为,综合力学性能优异。
图1
图1
Fe-15Mn-5Si-14Cr-0.2C铸态和加载断裂后试样XRD谱
Fig.1
XRD spectra of as-cast Fe-15Mn-5Si-14Cr-0.2C sample and fractured sample after loading
图2
图2
Fe-15Mn-5Si-14Cr-0.2C铸态试样室温工程应力-应变曲线
Fig.2
Engineering stress-strain curve of as-cast Fe-15Mn-5Si-14Cr-0.2C sample at room temperature
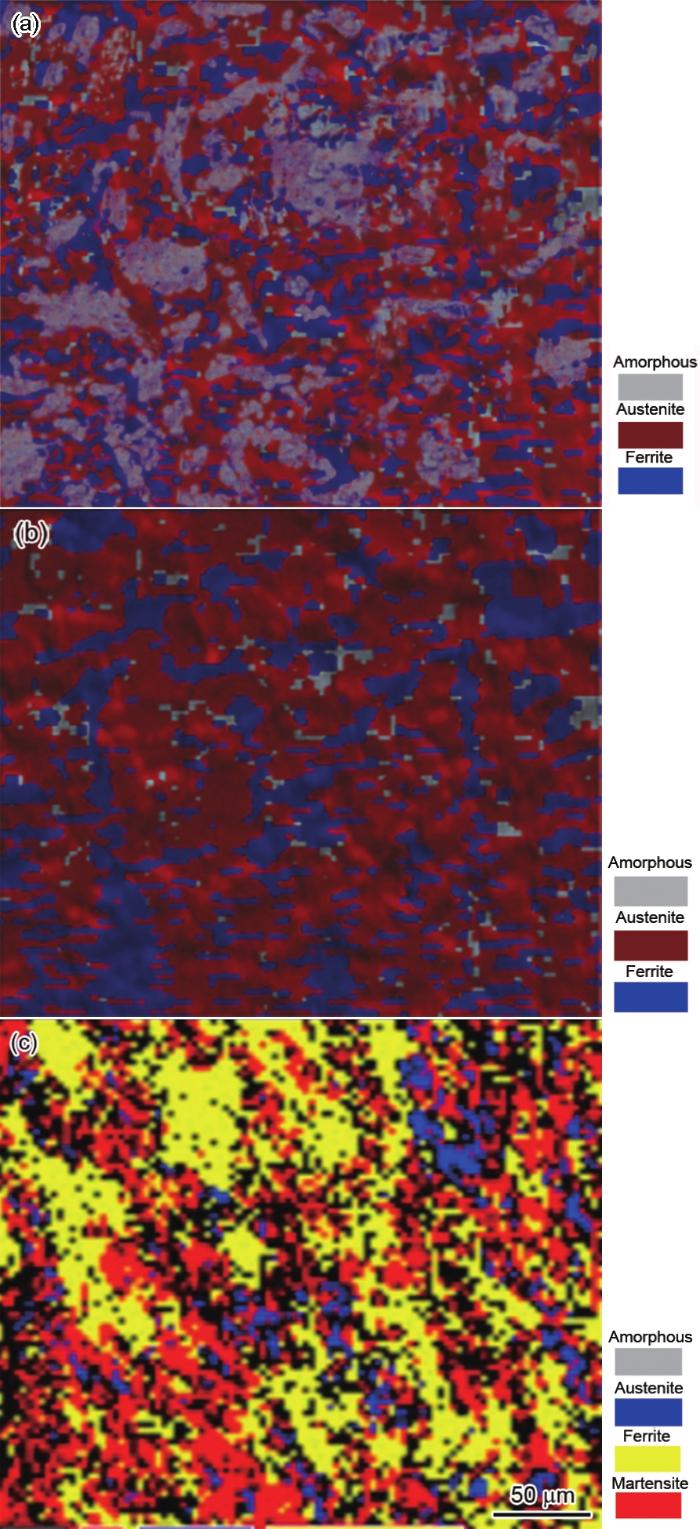
图3为Fe-15Mn-5Si-14Cr-0.2C铸态试样和加载断裂后横截面区域的EBSD像。如图3a所示,铸态试样边缘区域非晶相含量较多;图3b显示铸态试样中心区域晶体相含量较多,其中铁素体面积分数为49.53%,奥氏体相面积分数为21.04%,非晶相面积分数为29.43%。由于在吸铸冷却过程中,试样表面与铜模壁接触,热量传递快,使试样由表及里冷却速率降低,因此在温度梯度作用下形成了边缘非晶相含量较多,心部区域非晶相、奥氏体相和铁素体三相共存的梯度组织。另一方面,试样中心区域铁素体相大量析出,Cr元素含量较多,易于形成钝化膜,使试样的耐蚀性能增强[13]。而压应力加载断裂后,奥氏体部分转变为马氏体,如图3c所示,非晶相、残余奥氏体相、铁素体相和马氏体相四相共存,TRIP效应显著。
图3
图3
直径2 mm的Fe-15Mn-5Si-14Cr-0.2C铸态试样和断后试样横截面区域的EBSD像
Fig.3
EBSD images of as-cast Fe-15Mn-5Si-14Cr-0.2C sample in edge zone (a), center zone (b) and fractured sample after loading (c)
2.2 Fe-15Mn-5Si-14Cr-0.2C试样的腐蚀行为分析
图4
图4
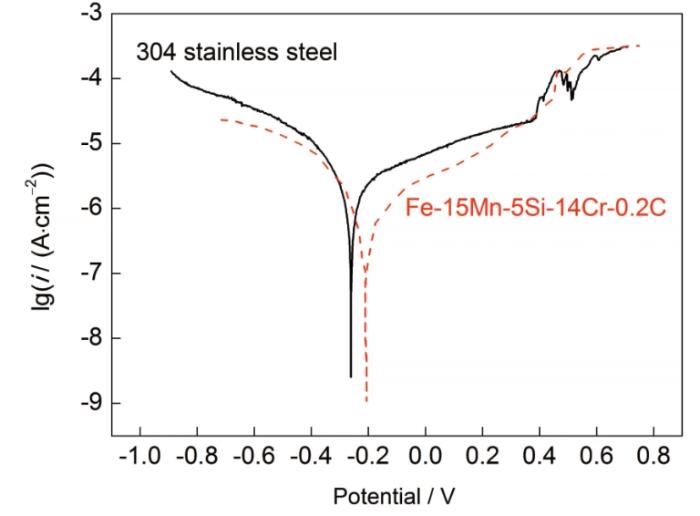
298 K下直径2 mm的Fe-15Mn-5Si-14Cr-0.2C试样与304不锈钢在人工海水中的极化曲线
Fig.4
Potentiodynamic polarization curves of Fe-15Mn-5Si-14Cr-0.2C alloy and 304 stainless steel in artificial seawater at 298 K (i—current density)
表1 298 K下直径2 mm的Fe-15Mn-5Si-14Cr-0.2C试样与304不锈钢在人工海水中的腐蚀参数
Table 1
Alloy | Ecorr mV | icorr μA·cm-2 | Rp 106 Ω·cm2 | Epit mV | Epit-Ecorr mV |
|---|---|---|---|---|---|
| 304 stainless steel | -263.43 | 1.582 | 2.2 | 384.93 | 648.41 |
| Fe-15Mn-5Si-14Cr-0.2C | -211.85 | 0.490 | 8.9 | 598.58 | 810.43 |
由Tafel直线外推法求得自腐蚀电流密度(icorr)。根据Faraday定律推导,可求得腐蚀速率(V)[14]:
式中,Δm为物质的量,S为横截面面积,t为通电时间,A为金属的原子量,n为金属的化合价,F为Faraday常数。由式(1)可知, V∝icorr,icorr可以反应V的大小[15]。
由Stern-Geary 方程算出极化电阻(Rp)[16]:
式中,βa和βc分别为阳极极化曲线和阴极极化曲线线性部分的斜率。Rp表示电荷在电极/溶液界面传递过程中单位面积上的等效电阻,Rp越大,icorr越小,腐蚀速度越慢[17]。由表1可知,Fe-15Mn-5Si-14Cr-0.2C试样与304不锈钢的自腐蚀电位分别为-211.85和-263.43 mV,Fe-15Mn-5Si-14Cr-0.2C试样的自腐蚀电位高不易失去电子,且钝化膜破裂电位(Epit)较高,钝化膜区间远大于304不锈钢,腐蚀倾向小于耐蚀性能已相当优异的304不锈钢。由图4可知,Fe-15Mn-5Si-14Cr-0.2C试样的极化曲线表现出良好的自钝化行为,结合表1可知,Fe-15Mn-5Si-14Cr-0.2C试样的钝化电流密度远小于304不锈钢,腐蚀速率低,Epit较高,表明试样在其表面生成的钝化膜更加稳定致密;自腐蚀电位与点蚀破裂电位差值(Epit-Ecorr)较大,其点蚀敏感性更低[18]。极化曲线在阳极极化区间出现波动后进入稳定状态,表明钝化膜的活化溶解与再生成同时进行,且修复能力远大于钝化膜的破裂过程并达到动态平衡状态使得曲线再次稳定。这是由于元素中原子种类和结构不同,主、次量子数也不同,导致Fermi能级不同,而Fermi能级越高,元素电极电位降低。就越易失去外层电子,发生电化学腐蚀。随原子序数增大,电极电位逐渐降低。Fe-15Mn-5Si-14Cr-0.2C试样中金属元素Fe和Cr的标准电极电位分别为-0.44和-0.71 V,其Fermi能级分别为-11.1×10-19和-6.93×10-19 eV。Cr元素电极电位较低,极易失去外层电子,在人工海水中最先反应形成Cr2O3氧化膜。由于试样含有大量的Fe-Cr铁素体相,而铁素体极易自钝化,形成致密的氧化膜;另一方面,试样表面非晶相较多,由于非晶相独特的原子结构,晶界较少,缺陷少,铁素体与非晶相的协同作用可提高试样的耐蚀性能。试样中添加Cr原子可增强纯Fe的强键键络,使相界面更为稳定,为Cr原子扩散到界面处改善钝化膜性能提供有利条件[19]。Fe-Cr固溶后形成的混合晶胞稳定性好,键能最高可达 70.3102 kJ/mol,远高于纯Fe主干键络键能(60 kJ/mol),稳定性比纯Fe好[20]。根据EET理论[21],最强键共价电子数越少,新相结构越容易形成。电子密度是衡量界面结合强度的标准,Cr2O3的界面电子密度(ρ)较高,可由共价电子数(n')和等效共价键数(I)及S求得[22]:
由于Cr电极电位较高,属于n型半导体,随着实验进行,钝化膜处于不断的破裂和修复状态中[23]。氧化膜生成与溶解的反应方程式为:
当生成-溶解速率相等时,反应达到平衡,形成稳定的氧化膜,从而阻止了Fe的阳极溶解反应:Fe→Fe2++2e[24]。
图5
图5
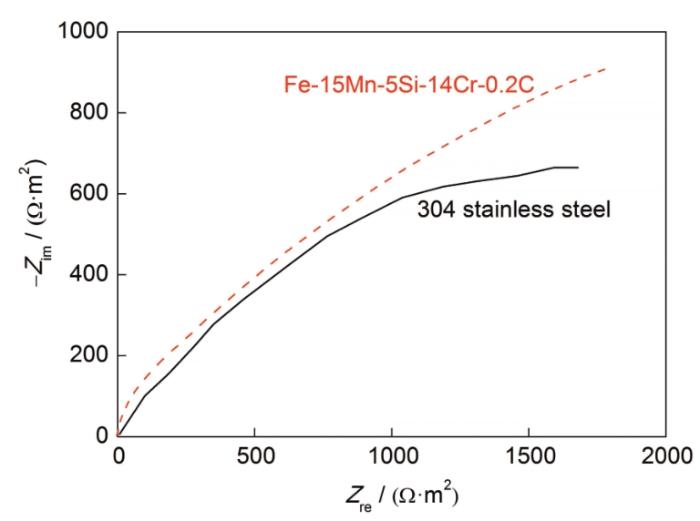
Fe-15Mn-5Si-14Cr-0.2C试样与304不锈钢在人工海水中的交流阻抗图
Fig.5
AC impedance diagrams of Fe-15Mn-5Si-14Cr-0.2C alloy and 304 stainless steel in artificial seawater at 298 K (Zim—imaginative part of impedance, Zre—real part of impedance)
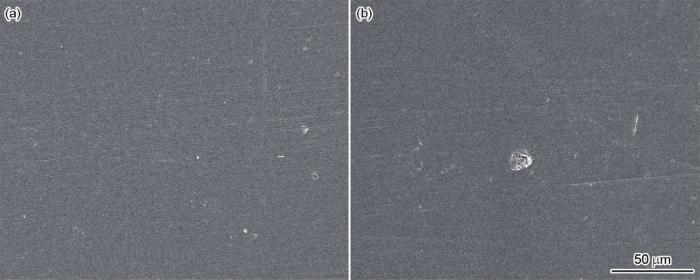
图6为Fe-15Mn-5Si-14Cr-0.2C非晶合金在人工海水介质中腐蚀后不同区域的SEM像。由图可见,试样边缘区域无明显腐蚀现象(图6a),试样中心区域仅局部有轻微点蚀出现(图6b)。试样中心区域EDS图显示Cr元素和O元素分布较为密集,而试样边缘区域EDS图显示Cr元素和O元素分布较少。表2为试样不同区域EDS面扫分析结果。由于试样边缘区域非晶相多,而非晶组织无晶界和其它晶体缺陷,耐腐蚀性能优异;中心区域Cr元素含量较多,与腐蚀介质接触后,O原子吸附到Fe-Cr合金表面,更易与Cr作用形成Cr2O3钝化膜,并且由于Cr2O3的电子密度差和杂化轨道数比Fe2O3大2个数量级,Cr原子更易偏离平衡位置与O元素结合形成Cr2O3保护性钝化膜,改变钝化膜结构,与非晶相相互协同,使试样更加耐蚀[26]。Cr元素在合金表面增多,由于非晶合金的热稳定性处于亚稳态,且合金组元的类金属元素活性较大,在腐蚀液中快速溶解使Cr元素在合金表面高度富集,导致迅速形成稳定且致密的钝化膜,使试样更快地进入钝化状态[27]。
图6
图6
Fe-15Mn-5Si-14Cr-0.2C试样腐蚀后边缘区域和中心区域的SEM像
Fig.6
SEM images of Fe-15Mn-5Si-14Cr-0.2C alloy after electrochemical corrosion at margin (a) and center (b) areas
表2 Fe-15Mn-5Si-14Cr-0.2C合金腐蚀后边缘区域和中心区域的EDS分析结果 (mass fraction / %)
Table 2
| Area | Fe | Mn | Si | Cr | C |
|---|---|---|---|---|---|
| Margin | 56.54 | 7.32 | 15.49 | 6.15 | 14.50 |
| Center | 58.80 | 11.60 | 6.58 | 8.27 | 14.75 |
由于人工海水中卤离子多种多样,包括Cl- (浓度为0.747 mol/L)、F- (浓度为0.8 mmol/L)、Br- (浓度为0.07 mmol/L),这些活性阴离子会优先迁移吸附在钝化膜薄弱处而发生点蚀。中心区域点蚀的出现是由于过饱和的C与Cr、Fe结合形成复杂的碳化物(CrFe)23C6,从CFe15.1过冷奥氏体相中析出而分布在晶界上。由于(CrFe)23C6中的Cr含量比晶内的平均Cr含量高很多,(CrFe)23C6的析出导致其周围晶界区消耗大量的Cr[28];而Cr在奥氏体中的扩散速率远低于C,使晶界区的Cr不能通过晶内Cr的扩散得到及时补充,导致晶界附近形成贫Cr区。由于贫Cr区的Cr含量低于不锈钢形成耐蚀钝化膜所需要的最低值,因而钝态受到破坏,晶粒与晶界及其附近区域形成大面积的钝化-活化微电池[29]。同时溶液中的Cl-吸附在钝化膜上,与氧化膜中的阳离子反应形成可溶性氯化物,使平衡受到破坏,溶解占优势,从而加速金属腐蚀。Cl-通过氧化膜的裂缝和蚀孔进入金属表面,当溶液浓度升高时,电极附近的Cl-浓度也随之升高,使Cl-在电极表面的吸附作用增强,更容易穿过电极表面钝化膜与金属基体结合,形成可溶性的络合物,如图6b所示,钝化膜破裂形成局部点蚀,这与前述极化曲线趋势相符[30]。
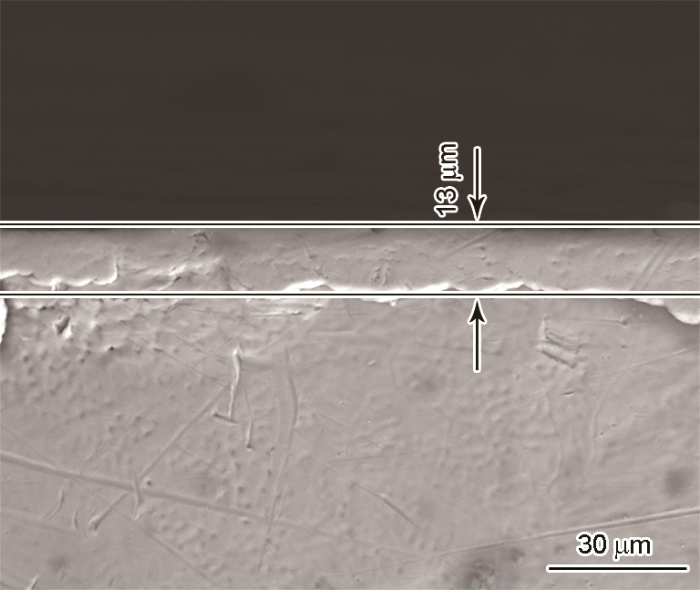
图7
图7
Fe-15Mn-5Si-14Cr-0.2C试样腐蚀后的SEM-BS像
Fig.7
SEM-BS image of Fe-15Mn-5Si-14Cr-0.2C after electrochemical corrosion
3 结论
(1) Fe-15Mn-5Si-14Cr-0.2C非晶合金组织是由非晶相+晶体相(CFe15.1过冷奥氏体相和Fe-Cr铁素体相)组成,边缘区域非晶相较多,心部区域非晶相、铁素体相和奥氏体相三相共存,且晶体相含量较多。室温压缩实验中,试样TRIP效应显著,表现出优异的综合力学性能,其屈服强度、断裂强度和塑性应变分别为978 MPa、2645 MPa和35.8%。
(2) Fe-15Mn-5Si-14Cr-0.2C非晶合金在人工海水中表现出良好的自钝化行为,与304不锈钢相比,Fe-15Mn-5Si-14Cr-0.2C试样的自腐蚀电位高,容抗弧大,自腐蚀电流密度小,且极化阻抗远大于304不锈钢;Fe-15Mn-5Si-14Cr-0.2C试样的腐蚀动力学速率低,钝化膜破裂区间大于304不锈钢,在腐蚀形貌边缘处未发现明显的点蚀和腐蚀产物,当氧化膜的生成与溶解达到动态平衡时,生成致密的Cr2O3氧化膜,且分布均匀,阻止了Fe的阳极溶解反应。
(3) Fe-15Mn-5Si-14Cr-0.2C非晶合金铸态组织中奥氏体相的存在,使得非晶复合材料综合力学性能更加优异;Fe-Cr铁素体与非晶相协同作用,使其耐腐蚀性能显著优于304不锈钢。由铸造过程的温度梯度制得的新型非晶复合结构钢,同时具备优异的力学性能和耐腐蚀性能,具有广泛的应用前景和工业价值。
参考文献
High strength bulk amorphous alloys with low critical cooling rates (Overview)
[J].
Shape memory bulk metallic glass composites
[J].
Structural heterogeneities and mechanical behavior of amorphous alloys
[J].
The nature and properties of amorphous matter
[J].
非晶态物质的本质和特性
[J].
Study of anticorrosion ability of Fe43.7Co7.3Cr14.7Mo12.6C15.5B4.3Y1.9 bulk metallic glass in strong acid solutions
[J].
FeCoCrMoCBY块体非晶合金在强酸介质中的耐蚀性能
[J].
Corrosion behavior of Fe41Co7Cr15Mo14C15B6Y2 bulk metallic glass in sulfuric acid solutions
[J].
Comparison of the corrosion of bulk amorphous steel with conventional steel
[J].
Corrosion-resistant amorphous Fe-C alloys containing chromium and/or molybdenum
[J].
Corrosion mechanism of N-containing Fe-Cr-Mo-Y-C-B bulk amorphous alloys in highly concentrated HCl solution
[J].
Influence of Cr additions on corrosion resistance of Fe- and Co-based metallic glasses and nanocrystals in H2SO4
[J].
Tribocorrosion behaviors of Inconel 625 alloy sliding against 316 steel in seawater
[J].
Microstructure and mechanical properties of welded joint of a Fe-Cr-Ni-Mo steel with high-strength and high-toughness
[J].
一种Fe-Cr-Ni-Mo高强高韧合金钢焊接接头的组织和力学性能
[J].
The electronic structure of passive films formed on stainless steels
[J].
Microsturcture and high temperature mechanical properties of martensitic stainless steel
[J].
1Cr12Ni2W1MolV马氏体不锈钢的组织和高温力学性能
[J].
腐蚀电化学原理
[M].
Electrochemical polarization I. A theoretical analysis of the shape of polarization curves
[J].
Improved corrosion resistance of novel Fe-based amorphous alloys
[J].With a continuously increasing aging population and the improvement of living standards, large demands of biomaterials are expected for a long time to come. Further development of novel biomaterials, that are much safer and of much higher quality, in terms of both biomedical and mechanical properties, are therefore of great interest for both the research scientists and clinical surgeons. Compared with the conventional crystalline metallic counterparts, bulk metallic glasses have unique amorphous structures, and thus exhibit higher strength, lower Young's modulus, improved wear resistance, good fatigue endurance, and excellent corrosion resistance. For this purpose, bulk metallic glasses (BMGs) have recently attracted much attention for biomedical applications. This review discusses and summarizes the recent developments and advances of bulk metallic glasses, including Ti-based, Zr-based, Fe-based, Mg-based, Zn-based, Ca-based and Sr-based alloying systems for biomedical applications. Future research directions will move towards overcoming the brittleness, increasing the glass forming ability (GFA) thus obtaining corresponding bulk metallic glasses with larger sizes, removing/reducing toxic elements, and surface modifications.
Tribocorrosion behaviors of a biodegradable Mg65Zn30Ca5 bulk metallic glass for potential biomedical implant applications
[J].
First principles calculation of occupancy, bonding characteristics and alloying effect of Cr, Mo, Ni in bulk α-Fe(C)
[J].
Cr, Mo, Ni在α-Fe(C)中占位、键合性质及合金化效应的第一性原理研究
[J].
Catalytic effect analysis of metallic catalyst during diamond single crystal synjournal
[J].
Electronic theoretical model of static and dynamic strength of steels
[J].
钢动静态强度计算的电子理论模型
[J].
Electronic theoretical study of the influence of Cr on corrosion resistance of Fe-Cr Alloy
[J].
Cr对Fe-Cr合金耐蚀性能影响的电子理论研究
[J].
Corrosion resistance of Fe-Cr-based amorphous alloys: An overview
[J].
Anodic dissolution and passiyation of an Fe-Ni base alloy in hot concentrated caustic solutions
[J].
Fe-Ni基合金在热浓碱溶液中的阳极溶解与钝化行为
[J].
Corrosion behaviors of bulk amorphous alloy Cu-Zr-Ti-Sn and its crystallized form in 3.5% NaCl solution
[J].
块状非晶合金Cu-Zr-Ti-Sn在3.5%NaCl溶液中的腐蚀行为
[J].
First-principles calculations of structure and magnetic properties of α-Fe(Si) phase precipitated in the Finemet alloy
[J].
Finemet合金析出相α-Fe(Si)结构与磁性的第一性原理计算
[J].
New Fe-based soft magnetic amorphous alloys with high saturation magnetization and good corrosion resistance for dust core application
[J].
Effect of chromium additions on the mechanical and physical properties and microstructure of Fe-Co-Ni-Cr-Mo-C ultra-high strength steel: Part I
[J].
Corrosion resistance of Fe-based amorphous alloys
[J]. J