随着人口膨胀,工业化进程的加快,环境污染越来越严重,环境污染防治己成为全球关注的重要课题[1]。自1972年Fujishima和Honda[2]发现TiO2电极在光照下分解水产生H2以来,光催化技术由于可以将低密度太阳能转化为高密度化学能,在紫外-可见光辐射下,矿化有机污染物、还原重金属离子、将温室气体CO2还原成可储存的CH4,发展成为解决当今社会能源和环境问题的关键技术之一。以TiO2为代表的宽带隙无机半导体材料的光催化性能被广泛研究。这类宽带隙半导体材料具有较高的光催化活性,但是由于对太阳能利用率低,限制了其在实际光催化过程中的应用[3,4]。例如,纳米TiO2的禁带宽度Eg=3.2 eV,可匹配的激发光源只能是波长<400 nm的紫外光,这部分光源只占太阳能辐射的5%,对太阳能中47%的可见光利用率很低。其它的一些窄带隙的无机半导体材料,如CdS、Fe2O3、Bi2O5、Ag2O等,虽然可利用可见光为激发光源,但是在光催化过程中存在着量子转换效率低、易发生光化学腐蚀等问题[5,6]。因此,探索开发新型的可见光催化体系,一直是光催化科学的研究热点和难点。
与氧化物和含氧酸盐比较,碳化物和氮化物由于C2p和N2p的轨道能级均高于O2p轨道能级,由C或N的2p轨道参与杂化构成的价带能级更高,在导带能级不变的前提下,带隙变窄,对可见光吸收增强。类石墨相g-C3N4是碳氮化物的典型代表[7,8]。单氰胺(H2NCN)是制备g-C3N4的前驱体[9,10],同时也是良好的有机配体,[NCN]2-是由三原子组成的线性离子,与N
1 实验方法
1.1 实验材料
实验所用材料为NiCl2·6H2O (纯度>98%)、Bi(NO3)3·5H2O (纯度>99.0%)、氨水(质量分数25%)、单氰胺水溶液(质量分数50%),以上试剂均为分析纯,使用前未进行处理。实验用水均为去离子水。
1.2 固相法合成Ni(HNCN)2/BiVO4
Ni(HNCN)2的制备:5 mmol的NiCl2·6H2O和0.025 mol H2NCN溶于5 mL的蒸馏水中,搅拌下向其中加入10 mL NH3·H2O,持续搅拌,慢慢形成绿色沉淀,去离子水洗涤3次,70 ℃下真空干燥12 h。
BiVO4的制备:0.008 mol Bi(NO3)3·5H2O溶解于80 mL乙二醇,0.008 mol NH4VO3溶解于80 mL热水。搅拌下,将NH4VO3溶液逐滴加入到Bi(NO3)3溶液中,混合溶液100 ℃下回流3 h,产生的黄色沉淀用水和乙醇溶液洗涤3次,70 ℃下真空干燥12 h。
Ni(HNCN)2/BiVO4复合颗粒合成:上述合成的Ni(HNCN)2和BiVO4按一定的摩尔比例加入到50 mL蒸馏水中,超声30 min,然后置于100 ℃的恒温水浴锅中将水蒸干。改变Ni(HNCN)2/BiVO4的摩尔比例,获得的复合颗粒命名为Ni(HNCN)2/BiVO4-x,x代表BiVO4相对于Ni(HNCN)2的摩尔分数,分别为0.3、1、2和3。
1.3 Ni(HNCN)2/BiVO4的结构表征
采用配备能谱(EDS)的SU8010冷场发射扫描电子显微镜(SEM)观察颗粒形貌和分析成分。应用D/max-2500PC X射线衍射仪(XRD,CuKα为线源,管电压50 kV,管电流100 mA,波长0.15406 nm)测定样品的XRD谱,扫描步长0.02°,扫描范围5°~70°。应用VERTEX 70红外光谱仪测定粒子Fourier变换红外 (FT-IR)光谱,KBr压片,扫描波数范围为4000~500 cm-1。应用Lambda 35紫外-可见分光光度计测定样品的紫外-可见(UV-Vis)漫反射光谱,波长范围200~800 nm。
样品Mott-Schottky曲线测定的实验过程为:称取1 mg光催化剂分散于0.8 mL水和0.2 mL乙醇混合溶剂,将20 μLNafion溶液加入到混合物溶液中,超声分散均匀。取80 μL上述溶液均匀涂覆在1 cm2的ITO导电玻璃上作为工作电极。应用CHI660E电化学工作站测定曲线,使用三电极体系(样品电极为工作电极,Pt丝为对电极,Ag/AgCl/KCl (3 mol/L)电极为参比电极),电解质溶液为0.2 mol/L的Na2SO4溶液。
1.4 罗丹明B的光催化降解实验
以罗丹明B为降解对象,测定Ni(HNCN)2 /BiVO4复合颗粒的光催化性能。100 mL的1×10-5 mol/L的罗丹明B溶液置于光催化反应容器中,加入50 mg 光催化剂,超声分散均匀。光照前,悬浮液在黑暗中磁力搅拌1 h以达到表面的吸附平衡。采用PLS-SXE300/300 UV型氙灯光源(300 W)为激发光源,420 nm滤光片截去紫外光。每隔一定光照时间,取5 mL悬浮液,离心分离后,上清液用紫外-可见分光光度计测定罗丹明B的吸光度,最大吸收波长为566 nm,根据工作曲线,确定染料的浓度变化。
罗丹明B的降解率(D)依照
式中,C0和C1分别为罗丹明B初始浓度和降解后的浓度。
2 实验结果
2.1 Ni(HNCN)2/BiVO4复合颗粒的结构
单一Ni(HNCN)2、BiVO4以及Ni(HNCN)2/BiVO4复合颗粒的形貌如图1所示。由图可见,Ni(HNCN)2为类纺锤形,颗粒长度约0.8 μm,纺锤状颗粒表面较光滑(图1a)。BiVO4颗粒类似棒状,平均直径约为1 µm,平均长度约为2.5 µm,棒状颗粒表面粗糙(图1b)。Ni(HNCN)2和BiVO4超声共混后得到Ni(HNCN)2/BiVO4-0.3、Ni(HNCN)2/BiVO4-2和Ni(HNCN)2/BiVO4-3复合颗粒的形貌如图1c、e和g所示。可见,棒状BiVO4颗粒的形貌没有发生改变,纺锤形Ni(HNCN)2颗粒尺寸明显变小,说明固相超声共混过程破坏了Ni(HNCN)2颗粒的形貌。较小的Ni(HNCN)2颗粒沉积在棒状BiVO4颗粒表面,形成异质结构。复合颗粒的EDS分别如图1d、f和h所示。可以看出,EDS中元素Ni和Bi的相对含量随着2种晶体复合比例的变化而改变。Ni(HNCN)2/BiVO4-0.3中元素Ni和Bi的原子数比为1∶0.33,Ni(HNCN)2 /BiVO4-2中Ni和Bi元素的原子数比为1∶2.45,与2种晶体的投入摩尔比接近,说明2种晶体混杂较为均匀,利于形成紧密接触的异质结构。Ni(HNCN)2/BiVO4-3 (图1h)中元素Ni和Bi的原子数比为1:7.06,与前2种复合颗粒相比较,2种晶体混合的均匀程度有所下降。
图1
图1
Ni(HNCN)2、BiVO4 和Ni(HNCN)2/BiVO4复合颗粒的SEM像和EDS分析
Fig.1
SEM images (a~c, e, g) and EDS analyses (d, f, h) of Ni(HNCN)2 (a), BiVO4 (b), Ni(HNCN)2/BiVO4-0.3 (c, d), Ni(HNCN)2/BiVO4-2 (e, f), Ni(HNCN)2/BiVO4-3 (g, h) (The inset in Fig.1a is the particles observed under higher magnification)
图2a为单一组分颗粒和Ni(HNCN)2/BiVO4复合颗粒的XRD谱。对于单一Ni(HNCN)2,其衍射峰归属于单斜晶系Ni(HNCN)2 (ICSD-172908)。对于单一BiVO4,其衍射峰对应于单斜晶系BiVO4 (JCPDS No.14-0688)。相比较,Ni(HNCN)2/BiVO4复合颗粒所有XRD峰可以归属于Ni(HNCN)2和BiVO4,且没有杂峰的出现,表明复合颗粒仅由Ni(HNCN)2和BiVO4 2种晶体构成。且XRD峰型比较尖锐,说明2种晶体晶化程度较高。与Ni(HNCN)2/BiVO4-0.3相对比,Ni(HNCN)2/BiVO4-2中随着BiVO4摩尔含量增加,Ni(HNCN)2衍射峰的相对强度减弱。
图2
图2
Ni(HNCN)2、BiVO4和Ni(HNCN)2/BiVO4复合颗粒的XRD谱和FT-IR谱
Fig.2
XRD spectra (a) and Fourier transform infrared spectroscopy (FT-IR) spectra (b) of single Ni(HNCN)2, BiVO4 and Ni(HNCN)2/BiVO4 composite
为了进一步研究2种晶体界面的作用方式,测定了复合颗粒的FT-IR光谱,如图2b所示。对比复合颗粒和单一组分粒子的IR光谱,Ni(HNCN)2/BiVO4复合颗粒的IR谱吸收峰表现为Ni(HNCN)2和BiVO4的IR吸收峰的叠加,没有新的IR吸收峰出现,证明Ni(HNCN)2和BiVO4之间是依靠物理相互作用结合形成复合颗粒。其中,3303 cm-1为—NH的伸缩振动峰,2214和1164 cm-1分别为[N—C≡N]2-的不对称伸缩振动峰和对称伸缩振动峰,639和569 cm-1分别为[N—C≡N]的变形振动峰和面外弯曲振动峰,1208 cm-1是—CNH的变形振动峰[17,22]。721 cm-1处的宽峰为BiVO4的V—O的振动吸收峰[23]。对比Ni(HNCN)2/BiVO4-0.3和Ni(HNCN)2/BiVO4-3的IR光谱,发现随着BiVO4的相对摩尔比例增加,Ni(HNCN)2的特征吸收峰相对强度减弱,BiVO4的特征吸收峰相对强度增加。
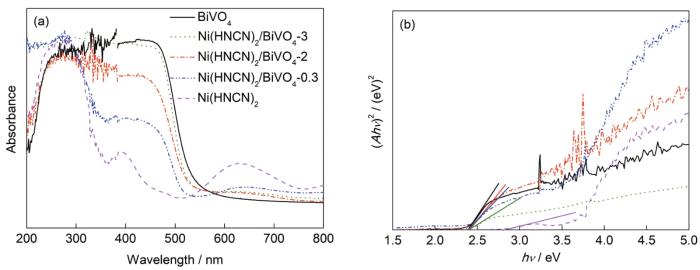
Ni(HNCN)2/BiVO4复合颗粒的UV-Vis漫反射吸收光谱如图3a所示。单一Ni(HNCN)2在紫外和可见区均有吸收,表现出较强的紫外-可见响应活性,其最大吸收峰分别位于290、390和630 nm。单一BiVO4在波长λ<530 nm出现强宽吸收峰。Ni(HNCN)2/BiVO4复合颗粒表现出2种组分的复合吸收峰,与Ni(HNCN)2相比,3个最大吸收峰的位置均发生一定程度红移,表明增强的可见光响应能力。
图3
图3
Ni(HNCN)2/BiVO4复合颗粒的UV-Vis光谱和(Ahν)2随 (hν)变化曲线
Fig.3
UV-Vis diffuse reflectance spectra of Ni(HNCN)2/BiVO4 (a) and re-plotted curves of (Ahν)2vs (hν) for Ni(HNCN)2/BiVO4 composites (b) (h—Planck constant, A—absorbance, v—frequency)
对于直接跃迁型半导体,其吸收带边的吸光度满足
式中,A表示吸光度,h为Plank常数,ν为入射光子的频率,Eg为禁带宽度,B为比例系数。将
2.2 Ni(HNCN)2/BiVO4复合颗粒的光催化性能研究
以罗丹明B为光催化降解对象,氙灯为激发光源,420 nm的截止滤光片滤去紫外光,测定了Ni(HNCN)2/BiVO4复合颗粒的光催化性能,结果如图4所示。从图4可以看出,单一的Ni(HNCN)2和BiVO4以及不同比例的Ni(HNCN)2/BiVO4复合颗粒在前60 min的静态吸附过程吸附量很小,吸附率不到6%。在之后的180 min的光催化降解实验中,单一的Ni(HNCN)2、BiVO4的降解率分别为24.1%和15.0%。复合颗粒对罗丹明B的降解率分别为:24.5% (Ni(HNCN)2/BiVO4-0.3)、24.1% (Ni(HNCN)2/BiVO4-1)、31.0% (Ni(HNCN)2/BiVO4-2)和21% (Ni(HNCN)2/BiVO4-3)。Ni(HNCN)2/BiVO4-2的降解率优于单一组分的Ni(HNCN)2和BiVO4以及其它比例制备的复合颗粒,这是因为Ni(HNCN)2与BiVO4之间形成良好的异质结构,两相界面的电子和空穴得到有效分离,光催化效率提高。
图4
图4
Ni(HNCN)2/BiVO4复合颗粒对罗丹明B的降解曲线
Fig.4
Photocatalytic degradation of Rhodamine B by Ni(HNCN)2 /BiVO4 composites (C—concentration at equilibrium, C0—concentration at initial, t—degradation time)
2.3 Ni(HNCN)2/BiVO4复合颗粒的能带结构及光催化机理
为了探究复合颗粒增强的光催化活性机理,确定复合颗粒的能带结构,测定了Ni(HNCN)2和BiVO4的Mott-Schottky (MS)曲线。如图5所示,Ni(HNCN)2和BiVO4的MS曲线的斜率为正,说明Ni(HNCN)2和BiVO4均为n型半导体。相对于Ag/AgCl/KCl (3 mol/L)参比电极,Ni(HNCN)2和BiVO4的平带电位(EFB)分别为-0.76和-0.48 V。根据文献报道,n型半导体导带电位(ECB)一般比平带电位低-0.1~-0.2 V[24~26];如果将参比电极替换为饱和氢电极,计算公式为ERHE=EAg/AgCl+0.059pH+E
图5
图5
Ni(HNCN)2和BiVO4颗粒的Mott-Schottky曲线
Fig.5
Mott-Schottky plots of Ni(HNCN)2 and BiVO4 particles (Csc—interfacial capacitance, E—electrode potential)
图6
图6
Ni(HNCN)2/BiVO4复合颗粒的能带结构示意图
Fig.6
Schematic of band structure of Ni(HNCN)2/BiVO4 composites (Eg—band gap energy)
3 结论
(1) 采用化学沉淀法制备了Ni(HNCN)2和BiVO4,并采用超声-固相共混法制备了不同比例的Ni(HNCN)2/BiVO4复合颗粒。与单一Ni(HNCN)2相比,Ni(HNCN)2/BiVO4复合颗粒的吸收峰位置发生红移,禁带宽度减小,禁带宽度变窄。通过电化学曲线以及UV-Vis吸收光谱确定了Ni(HNCN)2/BiVO4的能带结构。
(2) 以罗丹明B为降解对象,与单一BiVO4相比较,Ni(HNCN)2/BiVO4复合颗粒光催化降解效果均有所提高。其中,当Ni(HNCN)2与BiVO4摩尔比例为1∶2时,光降解效果最优。从复合颗粒能带结构可知,由于能带电势匹配,使得光生电子和空穴在两相界面有效流动,光催化效率提高。
参考文献
Eutrophication: A new wine in an old bottle?
[J].Eutrophication is one of the most common causes of water quality impairment of inland and marine waters. Its best-known manifestations are toxic cyanobacteria blooms in lakes and waterways and proliferations of green macro algae in coastal areas. The term eutrophication is used by both the scientific community and public policy-makers, and therefore has a myriad of definitions. The introduction by the public authorities of regulations to limit eutrophication is a source of tension and debate on the activities identified as contributing or having contributed decisively to these phenomena. Debates on the identification of the driving factors and risk levels of eutrophication, seeking to guide public policies, have led the ministries in charge of the environment and agriculture to ask for a joint scientific appraisal to be conducted on the subject. Four French research institutes were mandated to produce a critical scientific analysis on the latest knowledge of the causes, mechanisms, consequences and predictability of eutrophication phenomena. This paper provides the methodology and the main findings of this two years exercise involving 40 scientific experts.
Electrochemical photolysis of water at a semiconductor electrode
[J].
Nanoporous TiO2 spheres with tailored textural properties: Controllable synthesis, formation mechanism, and photochemical applications
[J].
A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis
[J].
Review on metal sulphide-based Z-scheme photocatalysts
[J].
Recent advances in BiVO4 semiconductor materials for hydrogen production using photoelectrochemical water splitting
[J].
Recent progress in g-C3N4 quantum dots: Synthesis, properties and applications in photocatalytic degradation of organic pollutants
[J]. J
Graphitic carbon nitride materials for photocatalytic hydrogen production via water splitting: A short review
[J].
A metal-free polymeric photocatalyst for hydrogen production from water under visible light
[J].
Comparative investigation on photoreactivity and mechanism of biogenic and chemosythetic Ag/C3N4 composites under visible light irradiation
[J].
Inorganic cyanamides. Physical and optical properties, and decomposition
[J].
Synthesis, crystal structure, and properties of MnNCN, the first carbodiimide of a magnetic transition metal
[J].
FeNCN and Fe(NCNH)2: Synthesis, structure, and magnetic properties of a nitrogen-based pseudo-oxide and -hydroxide of divalent iron
[J].
Synthesis, crystal-structure determination and magnetic properties of two new transition-metal carbodiimides: CoNCN and NiNCN
[J].Synthesis, structure determination, and magnetic properties are reported for the metastable and crystal-chemically isotypic phases cobalt carbodiimide, CoNCN, and nickel carbodiimide, NiNCN, adopting the hexagonal system and space group P63/mmc (NiAs type) with interatomic distances of Co-N = 2.17 Angstrom and Ni-N = 2.12 Angstrom and an octahedral coordination of the transition-metal ions; the NCN(2-) units reveal the carbodiimide shape with two C=N double bonds. The low-susceptibility data go back to strong antiferromagnetic spin-spin coupling, similar to the behavior of the electronically related oxides CoO and NiO.
Controllable synthesis of silver cyanamide as a new semiconductor photocatalyst under visible-light irradiation
[J]. J
Pseudoelementverbindungen. V. Pseudochalkogene—Versuch der empirischen und theoretischen Charakterisierung eines Konzeptes
[J]. Z
Fabrication of nanocomposites composed of silver cyanamide and titania for improved photocatalytic hydrogen generation
[J].
Fabrication of organic-inorganic hybrid membranes composed of poly (vinylidene fluoride) and silver cyanamide and their high photocatalytic activity under visible light irradiation
[J].
Structure and visible-light induced photocatalytic activity of zinc cyanamide-based photocatalysts
[J].2NCN/ZnNCN hetero structure has been also fabricated using the same ligand exchange process but mixing the silver salt with zinc salt together. Some means, such as XRD, SEM, infrared spectroscopy (FT-IR) and ultraviolet visible spectrometer (UV-Vis) were used to characterize the samples. The results showed that the single ZnNCN was flower-like particles with wide band gap (Eg=4.71 eV). Compared with single ZnNCN, the Ag2NCN/ZnNCN composite particles presented different morphology with rough surface, and physical interaction was existed between two kinds of metal cyanamide for Ag2NCN/ZnNCN composites. Because of the heterostructure, the light response spectrum for Ag2NCN/ZnNCN composite particles was extended to the visible light region, and the band gap was changed to 2.05 eV. The photocatalytic activity of Ag2NCN/ZnNCN composite particles in the degradation of Rhodamine B under Xenon irradiation was investigated, meanwhile, single ZnNCN and the mixture of Ag2NCN and ZnNCN was also applied in the photocatalysis under same conditions for comparison. The apparently enhanced photocatalytic activity of Ag2NCN/ZnNCN heterostructure was observed, and a first-order kinetic was discussed.]]>
基于氰胺锌的复合光催化剂的结构与可见光催化性能
[J].2NCN)/ZnNCN复合颗粒。利用XRD、SEM、红外光谱(FT-IR)和紫外-可见(UV-Vis)吸收光谱对光催化剂的结构进行表征。结果表明,单一ZnNCN为花瓣状颗粒,宽禁带半导体材料(禁带宽度Eg=4.71 eV)。Ag2NCN/ZnNCN复合颗粒形貌与单一ZnNCN和Ag2NCN相比有很大变化,2种金属氰胺化物以弱的物理作用力结合形成异质结构,复合颗粒的光谱响应范围扩展至可见光区,Eg=2.05 eV。以罗丹明B为光催化降解对象,研究了ZnNCN、Ag2NCN/ZnNCN复合颗粒在氙灯激发下的光催化活性。与单一ZnNCN以及Ag2NCN+ZnNCN机械混合物相比,Ag2NCN/ZnNCN复合颗粒表现出增强的光催化性能,表现为一级反应动力学特征。]]>
Synthesis and structure determination of Co(HNCN)2 and Ni(HNCN)2
[J].
Photocatalytic O2 evolution under visible light irradiation on BiVO4 in aqueous AgNO3 solution
[J].
Synthesis, structure determination, and quantum-chemical characterization of an alternate HgNCN polymorph
[J].
High IR reflecting BiVO4-CaMoO4 based yellow pigments for cool roof applications
[J].
Cobalt phosphide modified titanium oxide nanophotocatalysts with significantly enhanced photocatalytic hydrogen evolution from water splitting
[J].
Zinc vacancy-promoted photocatalytic activity and photostability of ZnS for efficient visible-light-driven hydrogen evolution
[J].
Visible-light-responsive 2D cadmium-organic framework single crystals with dual functions of water reduction and oxidation
[J].
Secondary branching and nitrogen doping of ZnO nanotetrapods: Building a highly active network for photoelectrochemical water splitting
[J].