奥氏体不锈钢经高温长时服役后,易脱溶析出M23C6碳化物。一方面,富Cr碳化物的析出和长大消耗了Cr元素,使得晶界附近出现贫Cr区,贫Cr区抵抗介质腐蚀的能力降低,造成了晶间腐蚀[9];另一方面,M23C6碳化物以及更长服役时间下析出的σ、χ、Laves等金属间相会造成延伸率、冲击韧性、持久强度等性能的下降[10~12]。为此,研究人员提出通过添加稳定化元素Nb来调控合金中的相析出行为,如316Nb、PNC316、HT-UPS、NF709等合金[13~16]。Nb与C的结合力远大于Cr,不仅可以有效抑制M23C6碳化物的形成,而且有利于细小弥散NbC的析出,能够起到有效钉扎位错运动的作用,从而提高奥氏体不锈钢的高温强度[13~15]。与此同时,细小弥散的NbC能够有效捕获辐照产生的缺陷,可降低肿胀行为[16]。然而,Nb添加会产生较大尺寸的初生NbC,初生NbC在后续的热加工、热处理过程中无法完全消除[17]。近年来,锆合金和Fe-Cr-Zr等合金的耐腐蚀性能研究[18~20]表明,第二相与基体的腐蚀行为可能存在差异,进而对合金的耐腐蚀性能产生影响。然而,初生NbC的腐蚀行为以及对奥氏体不锈钢耐腐蚀性能的影响尚不明晰,亟待开展相关的研究工作。
本工作以一种含Nb奥氏体不锈钢板材为研究对象,分别开展了550和600 ℃静态饱和氧液态Pb-Bi共晶(LBE)腐蚀实验,考察了LBE腐蚀过程中初生NbC的演化行为及其对氧化层形成的影响,从而揭示NbC的腐蚀行为及其对合金耐LBE腐蚀性能的影响机制。
1 实验方法
实验采用一种新型的Si增强奥氏体不锈钢,它是在316奥氏体不锈钢成分的基础上,提高了Si含量,添加了Nb,并适当调整其他合金元素含量[21]而得。Nb含量为0.9% (质量分数)的Si增强奥氏体不锈钢采用真空感应炉冶炼制备,铸锭经锻造、热轧成12 mm厚的板材。板材经1050 ℃固溶处理1 h,然后水淬。
在固溶态板材上切取尺寸为10 mm × 11 mm × 12 mm的样品,样品经SiC砂纸打磨至2000号、无水乙醇清洗后,置于具有饱和氧浓度的静态LBE中分别进行550和600 ℃下腐蚀实验。腐蚀实验采用自行搭建的LBE腐蚀实验装置[22],550 ℃下的腐蚀时间分别选取50、100、500、1000和5000 h,600 ℃下的腐蚀时间分别选取500和3000 h。
LBE腐蚀实验后,保留样品表面残留的Pb-Bi合金以保护氧化层不受破坏,采用冷镶嵌制备样品,并经研磨、抛光和清洗处理。采用MIRA 3型场发射扫描电镜(SEM)及其附带的Ultim MaxN型能谱仪(EDS)观察氧化层的截面形貌。采用EPMA-1600型电子探针(EPMA)分析氧化层的元素分布。采用聚焦离子束(FIB)切割技术制备目标微区的透射电镜(TEM)样品,随后在Talos场发射TEM上进行观察。利用体积比为1∶1∶1的CH3COOH + C2H5OH + H2O2溶液溶解掉试样表面残留的Pb-Bi合金后,采用SEM观察氧化层的表面形貌。采用D/max-2400PC型X射线衍射仪(XRD)分析氧化层的物相组成,测试条件为:CuKα,扫描步长0.02°,扫描角度2θ = 20°~80°。采用ESCALAB 250型X射线光电子能谱仪(XPS)分析表面氧化产物的元素价态,AlKα,测试电压3 kV,电流2 μA,溅射面积300 μm × 300 μm。
2 实验结果
2.1 微观组织
图1
图1
固溶态含Nb奥氏体不锈钢样品微观组织的SEM像
Fig.1
Low (a) and high (b) magnified SEM images of solution-treated Nb-containing austenitic stainless steel
2.2 550 ℃饱和氧LBE中的腐蚀行为
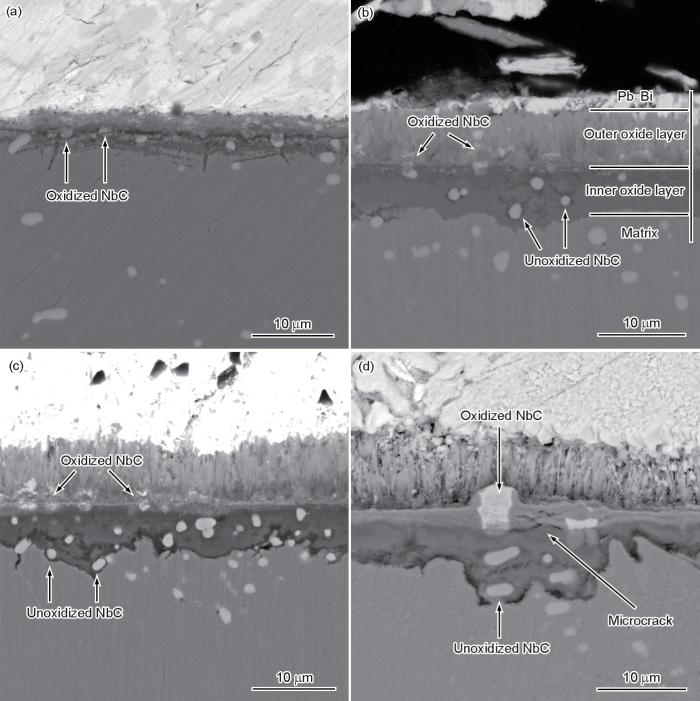
图2为固溶态样品经550 ℃饱和氧LBE腐蚀100、500、1000和5000 h后氧化层截面的背散射电子(BSE)像。腐蚀100 h后,样品表面形成了较薄的氧化层,厚度约为5 μm (图2a)。通过NbC与基体之间较强的对比度可分辨出NbC形貌,位于氧化层中的部分NbC形貌变得不规则(图2a中箭头所示)。腐蚀时间延长至500 h后,氧化层厚度达到了11 μm,呈现典型双层结构的氧化层,包括外氧化层和内氧化层(图2b)。借助NbC的分布特征可判定外氧化层/内氧化层界面为样品原始表面,这与O同位素示踪实验报道结果[23]一致。位于样品原始表面的NbC形貌变得不规则,体积轮廓变大,甚至出现在外氧化层中;而位于内氧化层的NbC保持完整的球状/椭球状形貌(图2b中箭头所示)。
图2
图2
固溶态样品经550 ℃饱和氧Pb-Bi共晶(LBE)腐蚀100、500、1000和5000 h后截面形貌的背散射电子(BSE)像
Fig.2
Backscattered electron (BSE) images of the cross-sectional morphologies of solution-treated Nb-containing austenitic stainless steel after exposure to oxygen-saturated lead-bismuth eutectic (LBE) at 550 oC for 100 h (a), 500 h (b), 1000 h (c), and 5000 h (d)
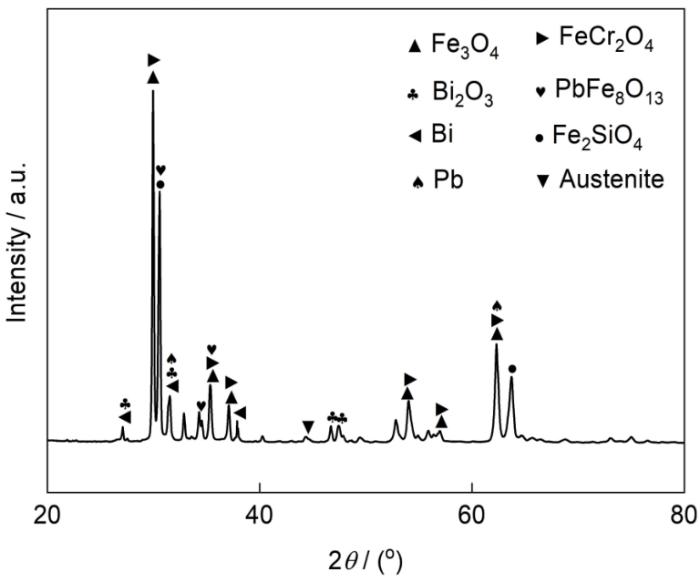
腐蚀5000 h样品表面残留的Pb、Bi被溶解后的XRD谱如图3所示。在XRD谱上仍可观察到残留Pb、Bi及其氧化物的微弱特征峰。可观察到奥氏体基体的特征峰,说明XRD谱获得了所有氧化物的特征峰。分析表明,样品表面形成的氧化物主要为Fe3O4和FeCr2O4,同时还有少量的Fe2SiO4和PbFe8O13。
图3
图3
固溶态样品经550 ℃饱和氧LBE腐蚀5000 h后的XRD谱
Fig.3
XRD spectra of solution-treated Nb-containing austenitic stainless steel after exposure to oxygen-saturated LBE at 550 oC for 5000 h
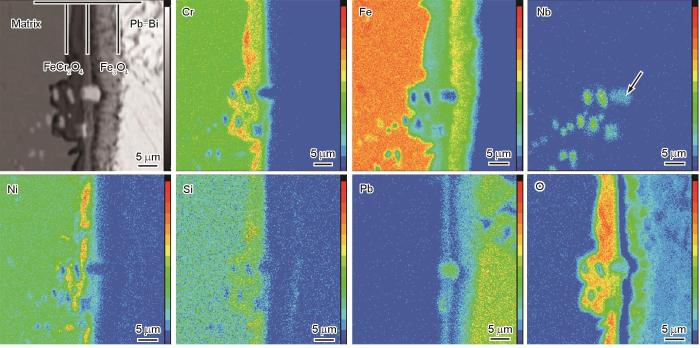
为确定NbC的腐蚀行为,进一步分析了图2d中对应位置的元素分布规律,EPMA结果如图4所示。由氧化层的元素分布特征可见,外氧化层富Fe而不含Cr,可观察到Pb的侵入;内氧化层富Cr、Si,贫Fe。结合XRD谱分析结果可知,外氧化层为Fe3O4,内氧化层为FeCr2O4和Fe2SiO4。Nb元素的分布规律可见,位于内氧化层的NbC中的Nb富集程度与基体中的NbC相当,保持完整的球状/椭球状形貌。与之不同的是,位于样品原始表面的NbC中的Nb富集程度明显降低,并且形貌变得不规则,部分Nb元素扩散至外氧化层中(图4中箭头所示),说明该位置处的NbC发生了氧化。此外,由Pb元素的分布规律可见,氧化态NbC中可观察到Pb的明显富集。
图4
图4
固溶态样品经550 ℃饱和氧LBE腐蚀5000 h后截面的EPMA元素分布
Fig.4
EPMA analyses of the cross-sectional area of solution-treated Nb-containing austenitic stainless steel after exposure to oxygen-saturated LBE at 550 oC for 5000 h (The black arrow indicates the oxidized NbC)
图5
图5
固溶态样品经550 ℃饱和氧LBE腐蚀5000 h后内氧化层中NbC附近的TEM分析结果
Fig.5
TEM analyses of the inner oxide scale of solution-treated Nb-containing austenitic stainless steel after exposure to oxygen-saturated LBE at 550 oC for 5000 h
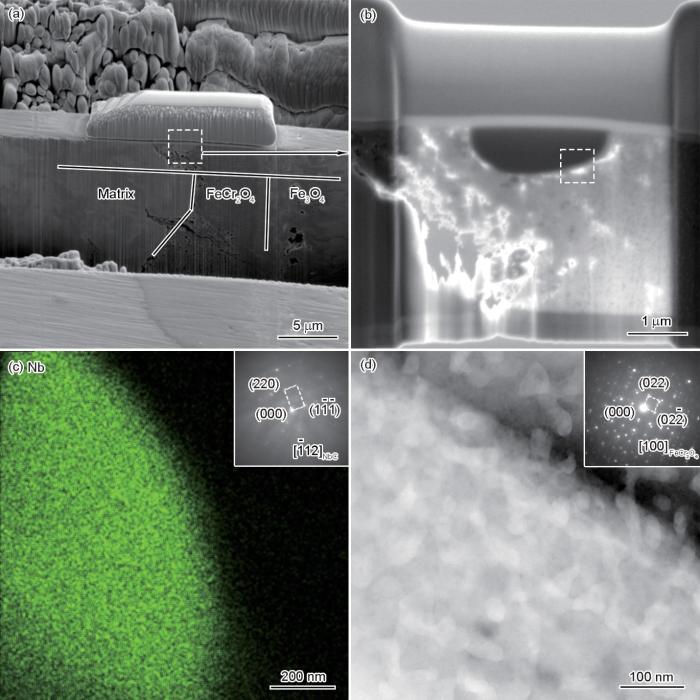
(a, b) BSE images of the FIB foil from inner oxide scale containing NbC
(c) EDS Nb mapping from the selected area in Fig.5b (Inset shows the SAED pattern of NbC)
(d) high-angle annular dark field (HAADF) image (Inset show the SAED pattern of oxide near NbC)
综上,550 ℃饱和氧LBE腐蚀条件下,位于材料内部的NbC未发生氧化,而位于样品原始表面的NbC易发生氧化。为了进一步分析位于样品原始表面NbC的早期腐蚀行为,对较短腐蚀时间(50 h)的样品进行表面形貌分析。腐蚀50 h样品表面残留Pb、Bi被溶解后的SEM像及EDS分析结果如图6a~c所示。SEM像显示,样品表面尚未完全被腐蚀产物覆盖,可分为未形成氧化层的区域和形成氧化层的区域(图6a)。在未形成氧化层的区域,仍可观察到呈带状分布的NbC (图6a中箭头所示)。高倍SEM像显示,NbC表面形成了氧化层,但出现了氧化层开裂现象(图6b)。EDS分析结果显示,氧化层为富Nb氧化物,结合元素含量结果初步推测氧化物为Nb2O5,同时氧化物中具有较高的Pb含量,说明Nb2O5无法有效阻碍LBE的侵入。
图6
图6
固溶态样品经550 ℃饱和氧LBE腐蚀50 h后表面形貌的SEM像、EDS及XPS分析结果
Fig.6
SEM surface morphologies (a-c) and XPS spectrum of Nb3d in surface oxide scale (d) in solution-treated Nb-containing austenitic stainless steel after exposure to oxygen-saturated LBE at 550 oC for 50 h
(a) low magnified SEM image
(b, c) high magnified SEM images of areas covered without (b) and with (c) oxide layer
2.3 600 ℃饱和氧LBE中的腐蚀行为
图7为固溶态样品经600 ℃饱和氧LBE腐蚀500和3000 h后氧化层截面的BSE像。可见,更高的腐蚀实验温度下,样品表面仍形成了双层结构的氧化层,但氧化层的生长速率显著加快。腐蚀500 h后的氧化层厚度约为20 μm (图7a),为550 ℃同一腐蚀时间下的1.8倍。与此同时,位于样品原始表面以及内氧化层的NbC形状变得不规则,说明均发生了氧化(图7a中箭头所示),且该区域形成了较厚的氧化层。腐蚀时间延长至3000 h后,氧化层厚度增加至54 μm,且内氧化层/外氧化层的界面不再平直(图7b)。发生氧化的NbC呈拉长形貌而向外扩散(图7b中箭头所示),氧化态NbC存在区域的氧化层显著变厚。
图7
图7
固溶态样品经600 ℃饱和氧LBE腐蚀500和3000 h后截面形貌的BSE像
Fig.7
BSE images of the cross-sectional morphologies of solution-treated Nb-containing austenitic stainless steel after exposure to oxygen-saturated LBE at 600 oC for 500 h (a) and 3000 h (b)
腐蚀3000 h样品氧化层截面的SEM像和EDS面扫描结果如图8所示。可见,外氧化层为富Fe的Fe3O4,Pb、Bi明显侵入到外氧化层中。内氧化层呈现出双层结构:靠近基体处形成了富Cr、Si的尖晶石氧化层;靠近外氧化层处的氧化层仅存在Cr的轻微富集,且观察到Pb、Bi的侵入。氧化态NbC存在于致密性较差的内氧化层中,随着NbC的不断氧化,Nb富集程度变得不明显。
图8
图8
固溶态样品经600 ℃饱和氧LBE腐蚀3000 h后截面形貌的SEM像和EDS面扫描图
Fig.8
SEM image and corresponding EDS mappings of the cross-sectional area of solution-treated Nb-containing austenitic stainless steel after exposure to oxygen-saturated LBE at 600 oC for 3000 h
3 分析讨论
3.1 NbC的氧化行为
550和600 ℃饱和氧LBE腐蚀实验结果表明,NbC可发生氧化,但氧化行为随温度的升高而发生变化。550 ℃下,NbC的氧化倾向与其在样品中的分布位置有关,位于样品原始表面的NbC易发生氧化,而位于样品内部的NbC则不会发生氧化。温度提高至600 ℃后,位于样品原始表面以及样品内部的NbC均会发生氧化。
基于普遍接受的“available space” LBE腐蚀模型[26],基体中的铁离子向外扩散与O2反应生成Fe3O4外氧化层,铁离子的外扩散会在基体中产生空位,空位聚集而产生微孔洞;与此同时,O2通过“纳米通道”快速扩散至微孔洞处,与基体中的Fe、Cr等反应而生成尖晶石结构的内氧化层,进而形成了双层结构的氧化层。其中,外氧化层、内氧化层形成的反应方程式分别表示为:
图9
图9
各种氧化反应的Ellingham-Richardson图
Fig.9
Ellingham-Richardson diagrams for possible oxides calculated by HSC chemistry 6.0
(a) Gibbs free energy of formation (ΔG)
(b) equilibrium oxygen partial pressure
腐蚀温度为550 ℃时,样品表面以及位于表面的NbC优先接触到具有饱和氧浓度的LBE,O浓度高于NbC氧化和Fe3O4生成的平衡氧分压,导致样品表面的NbC氧化。同时,NbC氧化反应的Gibbs生成自由能低于Fe3O4,与基体相比,NbC会优先与O2发生反应,因而可观察到腐蚀初期NbC优先氧化的现象(图6b)。随着氧化时间的延长,样品表面形成的氧化层可起到阻碍O向内扩散的作用,使得扩散进入基体中的氧分压降低,由此形成了更为致密的FeCr2O4内氧化层。然而,内氧化层中的氧分压尚未达到NbC氧化所需的平衡氧分压,因此位于内氧化层中的NbC不发生氧化。
腐蚀温度为600 ℃时,位于样品表面的NbC同样优先接触到具有饱和氧浓度的LBE而发生氧化。随着温度的升高,元素外扩散和O内扩散速率加快,内氧化层中的氧分压随之升高。同时,氧化层的致密性不如550 ℃形成的氧化层(图7),使其阻碍O向内扩散的作用减弱,也会增加内氧化层中的氧分压。因此,当氧分压达到NbC氧化的平衡氧分压时,便出现了内氧化层中NbC的氧化。
3.2 NbC氧化对氧化层形成的影响
由NbC的氧化反应式(3)可知,NbC氧化过程中,NbC转变为Nb2O5会发生体积变化,同时伴随着CO2气体的产生,均会对氧化层的形成过程产生影响,以下将从这2个方面进行分析。
此外,NbC转变为Nb2O5的同时,还会产生CO2气体。550 ℃下,仅位于样品原始表面的NbC发生了氧化,产生的CO2可逸出至周围环境中,其对氧化层形成的影响较小。600 ℃下,除位于样品原始表面的NbC外,位于样品内部的NbC也会发生氧化,在较大PBR带来的压应力以及氧化过程产生CO2的共同作用下,氧化层的致密性降低,使得氧化层的生长速率加快。因此,氧化态NbC所在的内氧化层中可观察到Pb、Bi的侵入,并且氧化层更厚(图8)。
4 结论
(1) 550 ℃饱和氧LBE腐蚀条件下,初生NbC的氧化倾向与其在样品中的分布位置有关,位于样品原始表面的NbC易发生氧化,而位于样品内部的NbC则不会发生氧化。温度提高至600 ℃后,位于样品原始表面以及样品内部的NbC均会发生氧化。
(2) 计算表明,同一温度下,NbC氧化反应的平衡氧分压介于外氧化层、内氧化层形成所需氧分压之间。550 ℃下,内氧化层中的氧分压尚未达到NbC氧化所需的平衡氧分压,是位于内氧化层中的NbC不发生氧化的原因;600 ℃下,氧分压达到NbC氧化的平衡氧分压,便出现了内氧化层中NbC的氧化。
(3) NbC氧化成Nb2O5后的体积膨胀为原来的2倍以上,是氧化态NbC体积轮廓变大的原因,可对周边区域的氧化层产生较大的压应力,导致氧化态NbC周围氧化层中出现微裂纹。
(4) NbC氧化伴随着CO2气体的产生,位于样品原始表面的NbC氧化产生的CO2可逸出至周围环境中,对氧化层形成的影响较小;而位于样品内部的NbC氧化产生CO2会降低氧化层致密性,使得氧化层的生长速率加快。
参考文献
Overview of lead-cooled fast reactor activities
[J].
Structural materials challenges for advanced reactor systems
[J].
Tailoring microstructure of austenitic stainless steel with improved performance for generation-IV fast reactor application: A review
[J].
Conventional austenitic steels as out-of-core materials for Generation IV nuclear reactors
[A].
δ-ferrite formation and its effect on the mechanical properties of heavy-section AISI 316 stainless steel casting
[J].
Role of δ-ferrite in fatigue crack growth of AISI 316 austenitic stainless steel
[J].
Temperature dependence of fracture behavior and mechanical properties of AISI 316 austenitic stainless steel
[J].
Effect of δ-ferrite on hot deformation and recrystallization of 316KD austenitic stainless steel for sodium-cooled fast reactor application
[J].
δ-铁素体对钠冷快堆用316KD奥氏体不锈钢热变形行为和动态再结晶的影响
[J].奥氏体不锈钢中δ-铁素体的存在显著影响热加工过程中奥氏体晶粒度的控制,造成晶粒不均匀现象,关于δ-铁素体对奥氏体动态再结晶行为的影响机制尚不清楚。本工作利用Gleeble-3800热力模拟试验机在1423 K、0.1 s<sup>-1</sup>条件下进行了铸态样品和均质化处理态样品的热压缩实验,结合SEM、EBSD和TEM等研究了δ-铁素体对热变形行为和动态再结晶的影响。结果表明:1473 K均质化处理14 h可基本消除δ-铁素体,同时伴随着奥氏体晶粒的显著长大,计算表明δ-铁素体向奥氏体的转变速率主要受控于Cr在奥氏体中的扩散。铸态样品变形过程中,δ-铁素体内部、δ-铁素体/奥氏体界面处的塑性变形先于奥氏体相,高温下较软δ-铁素体的存在,造成铸态样品的流变应力明显低于均质化处理态无δ-铁素体样品。随着变形的进行,δ-铁素体易发生动态回复,动态回复引起的软化导致流变应力显著下降。均质化处理态样品的动态再结晶机制是原始奥氏体晶界弓出形核的不连续动态再结晶。而δ-铁素体的存在促进了δ-铁素体/奥氏体界面附近奥氏体的动态再结晶,其机制为连续动态再结晶。铸态样品中的原始奥氏体晶界为不连续动态再结晶的形核位置,2种动态再结晶机制的耦合作用使得铸态样品的动态再结晶程度显著高于均质化处理态样品。
Determination of susceptibility to intergranular corrosion and electrochemical reactivation behaviour of AISI 316L type stainless steel
[J].
Precipitation in AISI 316L(N) during creep tests at 550 and 600 oC up to 10 years
[J].
Evolution of secondary phases in austenitic stainless steels during long-term exposures at 600, 650 and 800 oC
[J].
A short review on wrought austenitic stainless steels at high temperatures: Processing, microstructure, properties and performance
[J].
Understanding sigma-phase precipitation in a stabilized austenitic stainless steel (316Nb) through complementary CALPHAD-based and experimental investigations
[J].
Fatigue and creep-fatigue deformation of an ultra-fine precipitate strengthened advanced austenitic alloy
[J].
Creep behavior of Alloy 709 at 700 oC
[J].
Irradiation creep of 11Cr-0.5 Mo-2W, V, Nb ferritic-martensitic, modified 316, and 15Cr-20Ni austenitic S.S. irradiated in FFTF to 103-206 dpa
[J].
Homogenization temperature dependent microstructural evolution and mechanical properties in a Nb-stabilized cast austenitic stainless steel
[J].
Oxidation behaviour of zirconium alloys and their precipitates—A mechanistic study
[J].
Second phase particles and their corrosion behavior of Zr-0.72Sn-0.32Fe-0.15Cr-0.97Nb alloy
[J].Zr-0.72Sn-0.32Fe-0.15Cr alloy, which has much better corrosion resistance than that of Zr-4 alloy, was alloying by adding 1%Nb (mass fraction). In order to understand the effect of Nb on the corrosion resistance of Zr-0.72Sn-0.32Fe-0.15Cr alloy, the second phase particles (SPPs) and their oxidation behavior in this alloy were investigated using TEM, SEM and EDS techniques. Thin foil specimens for TEM observation were prepared for Zr-0.72Sn-0.32Fe-0.15Cr-0.97Nb alloy after recrystallization annealing. The corrosion tests for these thin foil specimens were conducted in an autoclave at 300 ℃, 8 MPa in deionized water for short time exposure. The results showed that a thin oxide layer in several hundred nanometers mainly consisted of the monoclinic ZrO2 formed on the surface, and SPPs embedded in the thin foil specimens at different corrosion levels were observed after corrosion test. The sizes of SPPs were mainly distributed between 30~150 nm and the maximum size was 230 nm. The size and crystal structure of SPPs have a relationship with Nb/Zr ratio (atomic ratio) of their composition. When Nb/Zr ratio was about zero, the size of SPPs was over 150 nm. When Nb/Zr ratio was in the range of 0.10~0.50, the sizes of SPPs were between 60~150 nm. When Nb/Zr ratio were in the range of 0.50~0.75, the sizes of SPPs were between 30~60 nm. When Nb/Zr ratio was over 0.75, the size of SPPs was smaller than 30 nm. With the increase of Nb/Zr ratio of their composition, three kinds crystal structure of Nb-containing SPPs, fcc (lattice constant a=0.701 nm), hcp (lattice constants a=0.508 nm, c=0.832 nm) and bcc (a=0.325 nm) structures were detected. SPPs without Nb containing have fcc structure (a=0.817 nm) while Fe/Cr ratio was over 5.00 and hcp structure (a=0.492 nm, c=0.788 nm) while Fe/Cr ratio was less than 3.00. The oxidation behavior of SPPs also had a relationship with the Nb/Zr ratio. The SPPs were easy to be oxidized to amorphous when Nb/Zr ratio was over 0.50. However, the SPPs with Nb/Zr ratio less than 0.50 were difficult to be oxidized.
Zr-0.72Sn-0.32Fe-0.15Cr-0.97Nb合金中的第二相及其腐蚀行为
[J].
Oxidation behavior of intermetallic phase and its contribution to the oxidation resistance in Fe-Cr-Zr ferritic alloy
[J].
Research advance on liquid lead-bismuth eutectic corrosion resistant Si enhanced ferritic/martensitic and austenitic stainless steels
[J].Structural materials are one of the major factors that restrict the lead-cooled fast reactor construction due to metallic elements that can dissolve in the liquid lead-bismuth eutectic (LBE), which may affect the structure's safety. T91 steel and 316 stainless steel are the leading structural materials for critical equipment such as fuel cladding, reactor vessels, and reactor core internals. The environmental compatibility of those steels with the liquid LBE needs to be systematically evaluated. However, T91 steel and 316 stainless steel suffer from rapid oxidation corrosion in oxygen-saturated LBE at 550oC. T91 steel's corrosion resistance in liquid LBE can be improved by decreasing the oxygen concentration (1.26 × 10-6%, mass fraction), but dissolved corrosion occurred at dissolved oxygen concentration below 1 × 10-6% for T91 steel and 316 stainless steel. T91 steel is sensitive to liquid metal embrittlement, significantly reducing its corrosion fatigue life in the liquid LBE. Compared to the standard (9%-12%)Cr ferritic/martensitic steel and 316 stainless steel, the microalloyed Si enhanced (9%-12%)Cr ferritic/martensitic steel (9Cr-Si and 12Cr-Si) and 316 stainless steel (ASS-Si) have good microstructural stability and comprehensive mechanical properties. The Si-rich oxide formation in liquid LBE improves the oxide film compactness and corrosion resistance. The dissolution corrosion was inhibited in static oxygen-saturation and oxygen-controlled (10-6%-10-7%) flowing liquid LBE (0.3 m/s) at 550oC for 9Cr-Si, 12Cr-Si, and ASS-Si. These alloys are expected to meet the design requirements for a lead-cooled fast reactor.
耐Pb-Bi腐蚀Si增强型铁素体/马氏体钢和奥氏体不锈钢的研究进展
[J].结构材料是制约铅冷快堆建设的关键因素之一,原因是其组成元素在液态Pb-Bi共晶(LBE)中会发生不同程度的溶解,影响结构安全。候选结构材料铁素体/马氏体钢T91与不锈钢316在550℃饱和氧LBE环境中发生快速氧化腐蚀;溶解氧浓度降至1.26 × 10<sup>-6</sup>% (质量分数)可减轻T91的液态LBE腐蚀,但低于1 × 10<sup>-6</sup>%时,T91与316钢发生溶解腐蚀;T91液态LBE脆化敏感性高,导致其在350℃液态LBE中腐蚀疲劳寿命显著降低。与商用的(9%~12%)Cr铁素体/马氏体钢和316型奥氏体不锈钢相比,经微合金化的Si增强型铁素体/马氏体钢(9Cr-Si和12Cr-Si)和奥氏体不锈钢(ASS-Si),具有较好的组织稳定性和综合力学性能,且在液态LBE中形成的富Si氧化物提高了氧化膜的致密性,改善了其耐腐蚀性能,在550℃下静态饱和氧和动态控氧LBE环境中的溶解腐蚀受到抑制,有望满足铅冷快堆的设计需求。
Oxide scale formation on ultrafine-grained ferritic-martensitic steel during pre-oxidation and its effect on the corrosion performance in stagnant liquid Pb-Bi eutectic
[J].
超细晶铁素体-马氏体钢的高温氧化成膜特性及其对Pb-Bi腐蚀行为的影响
[J].利用SEM、XRD、EPMA和XPS等研究了冷旋锻变形对9Cr2WVTa铁素体-马氏体钢在650℃空气中氧化膜形成过程的影响,在此基础上考察了预氧化制备氧化膜对9Cr2WVTa铁素体-马氏体钢在饱和氧液态Pb-Bi共晶 (LBE)中腐蚀行为的影响。结果表明,冷旋锻变形量的增加可提高样品的抗氧化性能,63%变形处理使抗氧化性能略有提高,94%变形处理可显著提高抗氧化性能。相较于回火态样品,63%变形处理样品形成的氧化物仍主要为(Fe, Cr)<sub>2</sub>O<sub>3</sub>,只是氧化物颗粒尺寸略有下降;94%变形获得的超细晶样品中,不仅氧化物颗粒尺寸显著减小,而且促进了富Mn氧化物(MnCr<sub>2</sub>O<sub>4</sub>和Mn<sub>2</sub>O<sub>3</sub>)的形成。超细晶样品中大幅度提高的空位浓度和位错密度、以及增加的晶界数量,显著提高了元素的扩散速率,Mn的异常快速扩散促进了富Mn氧化物的形成,稳定性好的富Mn氧化物提高了氧化膜的致密性。650℃空气中预氧化处理20 h后,超细晶样品表面获得致密性良好的富Mn氧化膜,在550℃饱和氧LBE中腐蚀500 h后,预氧化制备氧化膜可有效阻止LBE对铁素体-马氏体钢的侵蚀,并抑制了Fe的外扩散。Mn在LBE中较高的溶解度会加快富Mn氧化膜的溶解,随着Mn的不断溶解和LBE的持续侵蚀,预制备氧化膜的致密性不断下降而最终破裂,腐蚀2000 h后,样品表面形成了连续的Pb-Bi腐蚀产物。
Oxidation mechanism of a Fe-9Cr-1Mo steel by liquid Pb-Bi eutectic alloy (Part I)
[J].
Transmission electron microscopy study of complex oxide scales on DIN 1.4970 steel exposed to liquid Pb-Bi eutectic
[J].The deployment of Gen-IV lead-cooled fast reactors requires a good compatibility between the selected structural/cladding steels and the inherently corrosive heavy liquid metal coolant. An effective liquid metal corrosion mitigation strategy involves the in-situ steel passivation in contact with the oxygen-containing Pb-alloy coolant. Transmission electron microscopy was used in this work to study the multi-layered oxide scales forming on an austenitic stainless steel fuel cladding exposed to oxygen-containing (C-O approximate to 10(-6) mass%) static liquid lead bismuth eutectic (LBE) for 1000 h between 400 and 500 degrees C. The oxide scale constituents were analyzed, including the intertwined phases comprising the innermost biphasic layer.
Oxidation mechanism of a Fe-9Cr-1Mo steel by liquid Pb-Bi eutectic alloy (Part III)
[J].
Effect of silicon on the oxidation resistance of 9 wt.% Cr heat resistance steels in 550 oC lead-bismuth eutectic
[J].
Microstructures of oxidized primary carbides on superalloy Inconel 718
[J].
M23C6 precipitates induced inhomogeneous distribution of silicon in the oxide formed on a high-silicon ferritic/martensitic steel
[J].
Volume Ratio of an oxide to the metal
[J].
合金上氧化物的体积比的分析
[J].氧化物与形成该氧化物消耗的金属的体积比(Pilling-Bedworth Ratio简称PBR)是判断氧化膜完整性的一个重要判据,也是氧化膜内产生生长应力的主要因素之一,已发发表的关于PBR的数据都是针对纯金属的,本文基于合金的氧化行为建立了一个简化的模型,给出了合金上形成的氧化物的PBR的人体估算了Al2O3、NiO及Cr2O3等几种氧化物膜以及它们的混合膜PBR值,合金上氧化物的PBR值与合金 成分有关,并与纯金属上形成的同种氧化物的PBR有显著差别.
Pilling-Bedworth ratio for oxidation of alloys
[J].