面心四方(fct)结构的γ氢化物一般在较快的冷却速率、低H浓度条件下形成[9~11],在γ氢化物晶胞中,Zr与H原子占比接近1∶1[12,13],Zr原子占据晶胞的顶点和面心位置,H原子占据{110}晶面的4个四面体间隙[14]。Nath等[15]研究了H含量和冷却速率对氢化物相结构、形貌分布的影响,发现随H含量增加、冷却速率下降,γ氢化物占比逐渐减少。Long等[16]发现,在γ相之前会先形成H原子排列无序的γ'相,γ'相经时效之后转变为γ相。缓慢的冷却速率有利于形成fcc结构的δ氢化物[1,15,17,18],由于核燃料包壳在服役过程中冷却速率较低,因此δ相作为最常见的氢化物受到广泛关注。在δ氢化物中,Zr原子占据晶胞的顶点和面心位置,H原子随机占据fcc结构中的部分四面体间隙[19,20],H与Zr的原子比并非固定值,一般在1.5~1.7之间。Shiman和Daymond[21]研究表明,氢化物析出过程存在γ相向δ相转变的趋稳现象。δ相氢化物与Zr基体满足{0001}//{111}、<11
尽管冷却速率对氢化物析出的影响已有较多报道,但不同冷却条件下第二相粒子、氢化物应变在氢化物形核、宏观形貌形成过程中的作用,尚未见报道,仍需进一步澄清。因此,本工作利用光学显微镜(OM)、扫描电镜(SEM)等表征手段,研究不同冷却条件下析出氢化物的形貌、微观晶体学特征,探讨氢化物析出导致的应变对Zr基体以及其本身造成的微观结构变化,考察锆合金中第二相粒子对氢化物形核与生长的作用,最后结合第二相粒子以及应变分布实验结果对不同冷却条件下氢化物的析出和宏观形貌的形成进行分析讨论。
1 实验方法
实验所用材料为1.33 mm厚的Zr-4合金板材,名义成分为Zr-1.5Sn-0.2Fe-0.1Cr (质量分数,%),板材的轧制方向、横向和法线方向分别缩写为RD、TD和ND。从原材料板材切取40 mm × 60 mm × 1.33 mm (RD × TD × ND)的样品,使用砂纸打磨去除样品表面氧化层,随后在400℃纯H2气氛下开展充氢实验,最后将充氢样品在400℃下进行均匀化退火10 h,使用Inductar ONH cube型氧氮氢分析仪测量3个平行样品H含量,平均值为376 × 10-6。
从均匀化退火后的充氢样品上切割4个10 mm × 5 mm × 1.33 mm (RD × TD × ND)的块状试样,以5℃/min速率升温到400℃保温5 h,然后分别以0.5℃/min、炉冷、空冷和水淬等方式冷却至室温。对不同冷却条件下的样品进行机械抛光,使用5%HF + 45%HNO3 + 50%H2O (体积分数)的混合溶液腐蚀30 s,使用Axio Imager OM观察氢化物形貌与分布。使用5%HClO4 + 95%C2H5OH (体积分数)的混合溶液对样品电解抛光30 s,借助Gemini 460 SEM背散射电子(backscattered electron,BSE)、电子背散射衍射(electron backscattering diffraction,EBSD)表征氢化物微观形貌、相结构及其与基体的取向关系。将冷却条件为0.5℃/min的样品减薄至50 μm,使用10%HClO4 + 90%C2H5OH (体积分数)的混合溶液进行电解双喷,通过透射Kikuchi衍射(transmission Kikuchi diffraction,TKD)表征α-Zr基体中第二相粒子。
2 实验结果
2.1 Zr基体微观组织与织构
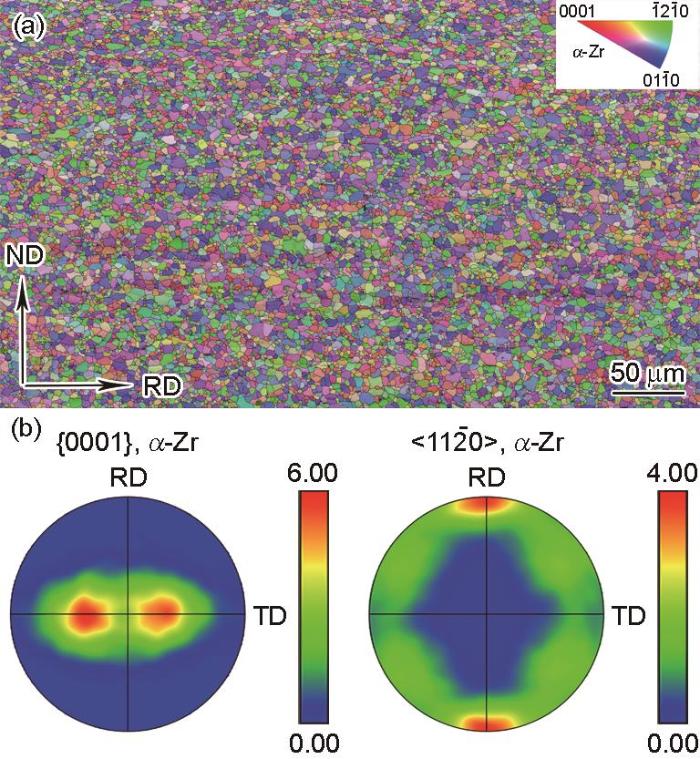
图1
图1
充氢和均匀化退火后Zr-4板材微观组织的EBSD结果
Fig.1
EBSD microstructure and texture of the Zr-4 plate after hydrogenation and homogenization annealing
(a) microstructure in the plane of normal direc-tion (ND) and rolling direction (RD)(b) {0001} <11
2.2 氢化物形貌与分布
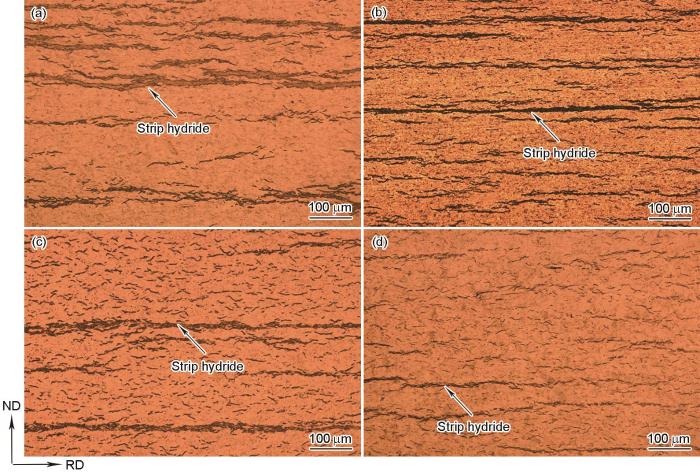
图2和3为不同冷却条件下Zr-4合金中氢化物的OM像。冷却条件为0.5℃/min时,氢化物在RD-ND截面呈现为粗大的长条状。长条状氢化物沿着RD生长,横贯整个观察区域,在ND方向平行分布,间隔几十到几百微米(图2a)。炉冷条件下,氢化物仍为长条状,但其厚度有所降低,在ND方向的氢化物间距降低,且在视野内观察到许多较短的条状氢化物,长度为100~200 μm (图2b)。随着冷却速率的增加,在空冷条件下析出的条状氢化物数量显著减少,在条状氢化物之间观察到许多细小的氢化物,它们大约几到几十微米长,均匀弥散地分布在Zr基体中(图2c)。随着冷却速率进一步增加,水淬样品中条状氢化物的长度和厚度进一步降低,细小氢化物遍布整个观察区域,氢化物整体分布更加弥散(图2d)。
图2
图2
不同冷却条件下Zr-4合金中氢化物的OM像
Fig.2
OM images of hydrides in Zr-4 alloy at various cooling conditions
(a) 0.5oC/min (b) furnace cooling (c) air cooling (d) water cooling
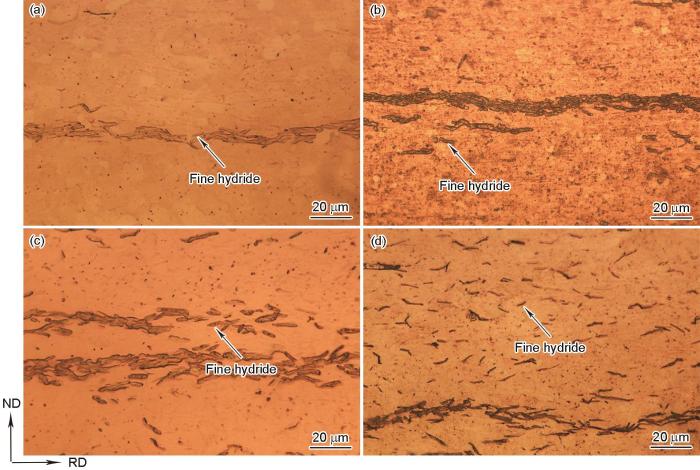
图3
图3
不同冷却条件下Zr-4合金中氢化物的高倍OM像
Fig.3
High magnification OM images of hydrides in Zr-4 alloy at various cooling conditions
(a) 0.5oC/min (b) furnace cooling (c) air cooling (d) water cooling
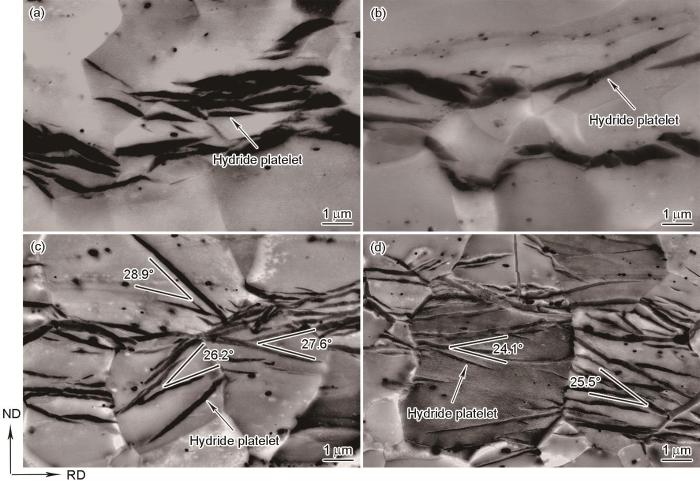
图4
图4
不同冷却条件下Zr-4合金中氢化物的BSE-SEM像
Fig.4
BSE-SEM images of hydrides in Zr-4 alloy at various cooling conditions
(a) 0.5oC/min (b) furnace cooling (c) air cooling (d) water cooling
2.3 氢化物相结构与取向关系
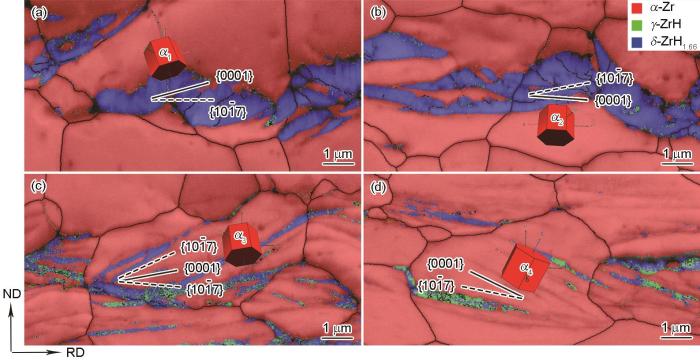
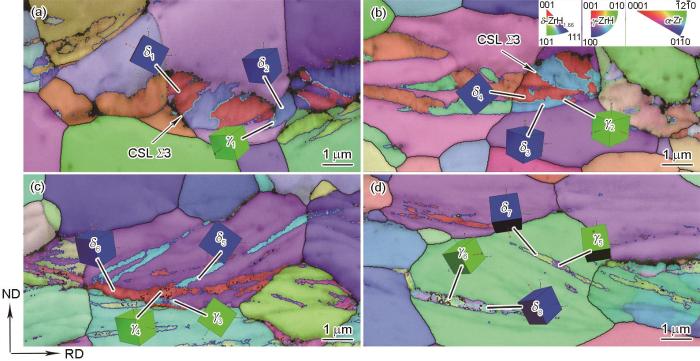
借助EBSD对氢化物相结构、取向关系进行表征分析。不同冷却条件下Zr-4合金内析出的氢化物的相分布图如图5所示,其中红色表示hcp结构的α-Zr基体,蓝色、绿色分别为fcc结构δ氢化物、fct结构γ氢化物。由图5a和b可知,缓慢冷却条件下氢化物以δ相为主。随着冷却速率增加,在空冷和水淬样品(图5c和d)中观察到更多γ相氢化物,同时注意到γ相氢化物并非独立存在,而是与δ相混合共存于氢化物片层中,因此判断γ与δ氢化物的析出相关联。根据α-Zr晶粒的三维晶胞示意图以及氢化物的生长方向,可以确定氢化物的析出惯习面为α-Zr基体的{10
图5
图5
不同冷却条件下Zr-4合金中氢化物的相分布图
Fig.5
Phase distribution maps of hydrides in Zr-4 alloy at various cooling conditions (The red hexagonal units indicate the crystallography orientation of corresponding zirconium grain)
(a) 0.5oC/min (b) furnace cooling (c) air cooling (d) water cooling
图6
图6
不同冷却条件下Zr-4合金中氢化物的反极图
Fig.6
Inverse pole figures (IPFs) of hydrides in Zr-4 at various cooling conditions (The blue and green cubes represent the crystallographic orientations of δ- and γ-phase hydrides, respectively)
(a) 0.5oC/min (b) furnace cooling (c) air cooling (d) water cooling
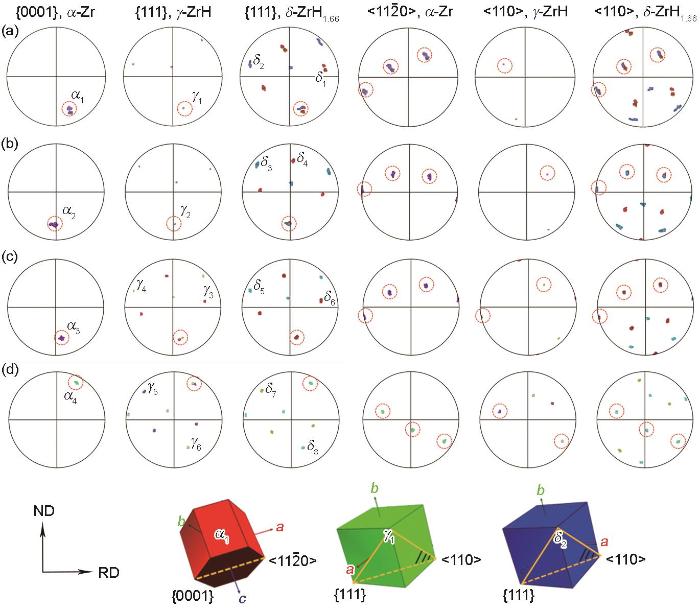
图7为4种冷却条件下析出的氢化物与其母体Zr晶粒的极图,占据极图中相同投影位置的晶面或晶向可视为相互平行。由此可以判断,γ、δ相氢化物与α-Zr基体均满足{0001}//{111}、<11
图7
图7
不同冷却条件下Zr-4合金中Zr晶粒与氢化物的极图
Fig.7
PFs of Zr grains and hydrides in Zr-4 alloy at various cooling conditions (The red, green, and blue unit cells represent the crystallographic orientations of α1, γ1, and δ2, respectively)
(a) 0.5oC/min (b) furnace cooling (c) air cooling (d) water cooling
2.4 第二相粒子的作用
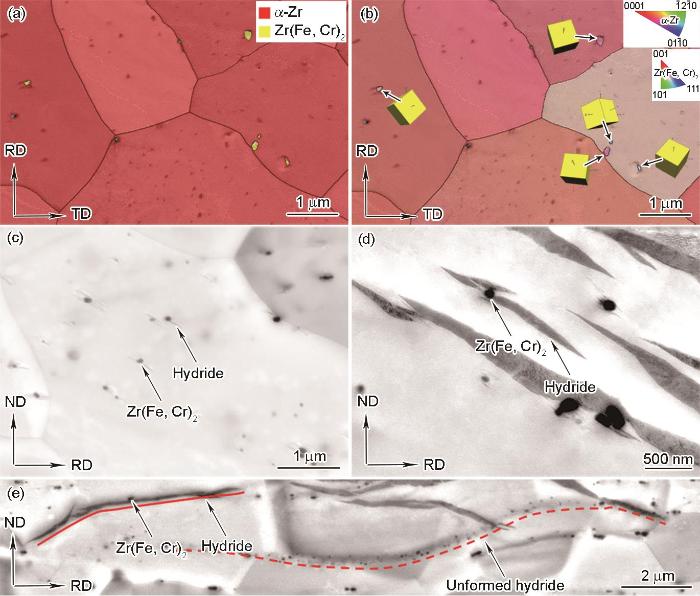
图8为0.5℃/min冷却条件下Zr-4合金内第二相粒子及其周围氢化物的相分布图、反极图和BSE-SEM像。已知Zr-4合金中第二相粒子主要为金属间化合物Zr(Fe, Cr)2,如图8a和b中TKD分析结果所示,黄色析出相为fcc结构的Zr(Fe, Cr)2。使用BSE-SEM观察可以发现,围绕第二相粒子析出的纳米氢化物在Zr(Fe, Cr)2颗粒两侧呈翼状分布(图8c),这些纳米氢化物能够生长成为血小板状氢化物,如图8d所示。在图8e中,红色实线位置展示了第二相粒子在氢化物相互连接、形成条状形貌过程中的促进作用,此外,观察到沿着红色虚线密集排列的Zr(Fe, Cr)2相,判断它们周围黑色衬度区域为埋藏在观察面内部的条状氢化物。考虑第二相粒子界面对H溶质原子具有陷阱捕获作用[27],因此Zr(Fe, Cr)2局部H浓度较高,在冷却过程中为氢化物提供了优先形核位点。缓慢冷却时,第二相粒子能够提高氢化物之间的连通性,促进条状氢化物生长达到数百微米的长度;在快速冷却时,第二相粒子可为氢化物提供晶内形核位点,促进氢化物在Zr晶粒内部析出。
图8
图8
0.5℃/min冷却条件下Zr-4合金内的Zr(Fe, Cr)2第二相粒子及其周围氢化物相图、反极图和BSE-SEM像
Fig.8
Phase distribution map (a), IPF (b), and BSE-SEM (c-e) images of Zr(Fe, Cr)2 second phase particles and their surrounding hydrides in Zr-4 alloy at 0.5oC/min cooling condition (Yellow cubes show the orientation of the second phase particles)
3 分析讨论
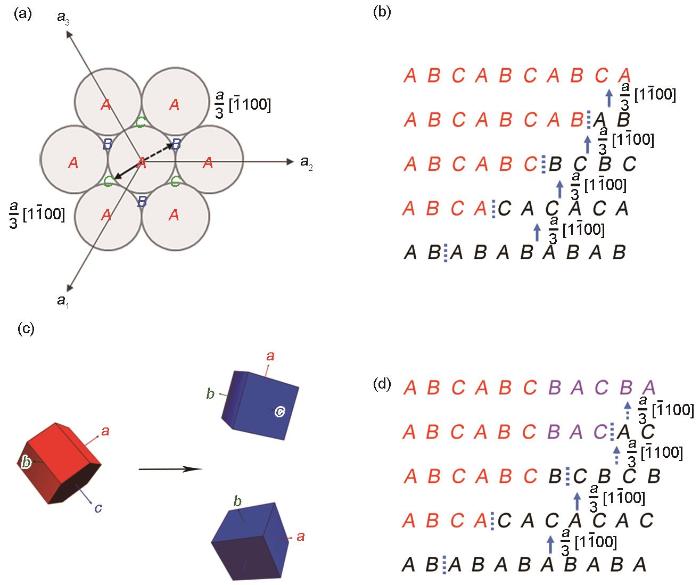
图9为氢化物与氢化物孪晶的形成示意图。hcp密排面堆垛结构中的A、B、C原子占位如图9a所示,Zr基体向fcc结构氢化物的转变通过Zr原子在{0001}平面上
图9
图9
锆合金中氢化物与氢化物孪晶形成示意图
Fig.9
Schematic illustrations of hydride and hydride twins formation in zirconium alloy
(a) three lattice occupations of atoms in hcp structure
(b) phase transformation of hcp-Zr into fcc-hydride
(c) 3D unit cells of α-Zr grain and hydride twins
(d) δ-hydride twin formation
Zr基体向氢化物转变过程中,
氢化物析出对Zr基体造成的应变可以通过以下公式来计算[29]:
式中,
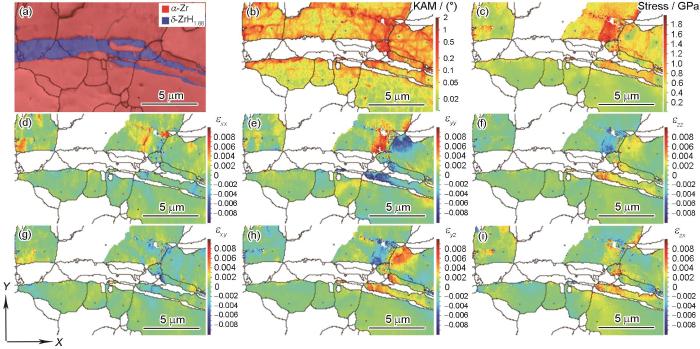
氢化物析出体积膨胀不仅约束自身长大,形成片层、长条状,同时对周围Zr基体造成应变,部分应变由氢化物孪晶缓解,部分由氢化物以及Zr基体内位错适配。图10为氢化物析出导致的应力场与应变场的高分辨EBSD (high-resolution EBSD,HR-EBSD)表征结果。图10a为所选区域的相分布图。图10b和c显示氢化物析出产生的应力应变主要分布在氢化物尖端以及氢化物与Zr基体的相界。从应变各向异性角度分析(图10d~f),氢化物尖端存在明显的垂直于片层厚度方向的拉伸应力,这能够吸收H溶质原子向尖端位置扩散、促进氢化物长大[6]。此外,剪切应变(图10g~i)有利于Zr基体{0001}平面上
图10
图10
0.5℃/min冷却条件下Zr-4合金内氢化物析出的应力与应变场
Fig.10
Stress and strain fields of hydride precipitation in Zr-4 alloy under 0.5oC/min cooling condition characterized by high-resolution electron backscattering diffraction (HR-EBSD) (εxx, εyy, and εzz are the normal stresses in three directions; εxy, εyz, and εzx are the shear stresses in three planes)
(a) phase map (b) HR-kernel average misorientation (KAM)
(c) von Mises stress (d-f) normal strains (g-i) shear strains
缓慢冷却条件下,扩散时间充足,H溶质原子可通过长程扩散至氢化物尖端,纳米氢化物在单个Zr晶粒内通过自催化形核堆垛、生长成为血小板状氢化物,不同Zr晶粒内的氢化物通过沿晶或穿晶生长、相互连接,从而形成数百微米长的条状氢化物。冷却速率越慢,能够完成长程扩散的H原子范围越广,形成的条状氢化物越粗大,间距也越宽。冷却速率较快时,受扩散限制,已生成的纳米氢化物周围H浓度无法满足氢化物继续生长的成分条件,Zr晶粒内纳米氢化物呈现多点形核情况,相邻Zr晶粒内的氢化物无法相互连接,故形成均匀弥散的分布形貌。
4 结论
(1) 随冷却速率增加,条状δ氢化物厚度和间距降低,形成细小弥散的氢化物分布形貌,微观上晶内氢化物数量增多,γ氢化物比例升高。
(2) 氢化物析出惯习面为Zr基体{10
(3) 证实氢化物内部存在{111}<11
(4) 氢化物的条状形貌与其析出导致的各向异性的应变密切相关。氢化物尖端的高应变,能够诱导新的纳米氢化物在已有氢化物尖端位置优先形核、生长,然后通过血小板氢化物堆垛、排列构成条状氢化物。此外,锆合金中Zr(Fe, Cr)2第二相粒子作为微区氢陷阱与形核位点,也对氢化物条状形貌的形成起到促进作用。
参考文献
A review on hydride precipitation in zirconium alloys
[J].
Design and mechanical characterization of a Zr-Nb-O-P alloy
[J].
Hydrogen in Zircaloy: Mechanism and its impacts
[J].
Modeling and simulation of hydrogen behavior in Zircaloy-4 fuel cladding
[J].
Multidimensional simulations of hydrides during fuel rod lifecycle
[J].
Hydrogen diffusion under stress in Zircaloy: High-resolution neutron radiography and finite element modeling
[J].
Experimental determination of zirconium hydride precipitation and dissolution in zirconium alloy
[J].
The effect of zirconium hydride on the corrosion and mechanical behavior of zirconium base metal: Experimental and simulation studies
[J].
The precipitation of zirconium hydride in zirconium and Zircaloy-2
[J].
Comments on the stability of zirconium hydride phases in Zircaloy
[J].
Evaluating zirconium-zirconium hydride interfacial strains by nano-beam electron diffraction
[J].
Some observations on the phase transformations in zirconium hydrides
[J].
Tetragonal hydride in low hydrogen content zircaloy
[J].
Evidence of stress-induced hydrogen ordering in zirconium hydrides
[J].
Effect of hydrogen concentration and cooling rate on hydride precipitation in α-zirconium
[J].
Identifying the true structure and origin of the water-quench induced hydride phase in Zr-2.5Nb alloy
[J].
Hydride formation by high temperature cathodic hydrogen charging method and its effect on the corrosion behavior of Zircaloy-4 tubes in acid solution
[J].
Characterization of zirconium hydrides in Zircaloy-4 cladding with respect to cooling rate
[J].
Neutron and X-ray diffraction studies on nonstoichiometric metal hydrides
[R].
Structure and thermodynamic properties of zirconium hydrides by structure search method and first principles calculations
[J].
Phase transformation and microstructural changes during the hydriding and aging processes in dual phase Zr alloy
[J].
EBSD studies on microstructure and crystallographic orientation of δ-hydrides in Zircaloy-4, Zr-1% Nb and Zr-2.5% Nb
[J].
Preferential precipitation of hydrides in textured Zircaloy-4 sheets
[J].
Microstructure and formation mechanisms of δ-hydrides in variable grain size Zircaloy-4 studied by electron backscatter diffraction
[J].
The habit planes of zirconium hydride in zirconium and zircaloy
[J].
{10
Evidence of hydrogen trapping at second phase particles in zirconium alloys
[J].Zirconium alloys are used in safety-critical roles in the nuclear industry and their degradation due to ingress of hydrogen in service is a concern. In this work experimental evidence, supported by density functional theory modelling, shows that the α-Zr matrix surrounding second phase particles acts as a trapping site for hydrogen, which has not been previously reported in zirconium. This is unaccounted for in current models of hydrogen behaviour in Zr alloys and as such could impact development of these models. Zircaloy-2 and Zircaloy-4 samples were corroded at 350 °C in simulated pressurised water reactor coolant before being isotopically spiked with HO in a second autoclave step. The distribution of H, Fe and Cr was characterised using nanoscale secondary ion mass spectrometry (NanoSIMS) and high-resolution energy dispersive X-ray spectroscopy. H was found to be concentrated around second phase particles in the α-Zr lattice with peak hydrogen isotope ratios of H/H = 0.018-0.082. DFT modelling confirms that the hydrogen thermodynamically favours sitting in the surrounding zirconium matrix rather than within the second phase particles. Knowledge of this trapping mechanism will inform the development of current understanding of zirconium alloy degradation through-life.
The precipitation of γ-zirconium hydride in zirconium
[J].
The dilatational misfit of zirconium hydrides precipitated in zirconium
[J].
Microstructural and crystallographic analysis of hydride reorientation in a zirconium alloy cladding tube
[J].
Hydride precipitation in α/β zirconium alloys
[J].
Autocatalytic nucleation and elastic stabilization of linear arrays of plate-shaped precipitates
[J].