在众多的磁可分材料中,石墨烯包覆过渡金属纳米颗粒TM@G (TM = Fe、Co、Ni)是一种非常具有潜力的磁可分催化剂载体,这种载体具有很强的可修饰性,不仅可以通过改变包覆金属种类和结构来改变载体表面的电子结构,也可以通过掺杂N、P原子的方式改变载体表面的配位环境,进而调控金属催化剂的催化性能[13~17]。Gao等[18]利用Ni的硝酸盐浸渍在炭黑上,在450℃下煅烧形成的C包覆Ni核壳结构催化剂对1-癸烯加氢反应获得最高加氢产率。Lv等[19]通过将Fe-Co2P核封装到N掺杂石墨烯壳内开发了一种二元金属掺杂核壳催化剂,发现Fe-Co2P核是协同催化活性中心,Fe-N-C作为有效催化活性位点。因此TM@G是一种可扩展的磁性载体,有望在液相催化加氢领域得到广泛应用。
本工作通过简单的煅烧方法制备磁可分的N掺杂的石墨烯包覆Ni颗粒载体(Ni@NG),通过传统的沉积-沉淀方法将金属Pt负载到Ni@NG载体上,得到具有磁可分性能的负载型Pt基催化剂(Pt/Ni@NG),以硝基苯加氢制苯胺作为探针反应,研究Pt/Ni@NG催化硝基苯加氢性能。结果表明,通过调控Pt 在Ni@NG 载体表面的负载量(质量分数分别为0.1%、0.3%和0.5%),可以实现Pt以单原子、团簇和纳米颗粒的形式分散在载体表面,其中以Pt团簇形式分散为主的0.3%Ptn/Ni@NG催化剂具有磁分离性能的同时,表现出优异的硝基苯加氢性能。
1 实验方法
1.1 Ni@NG载体制备
取2 g Ni(OH)2 (纯度99%)和6.368 g乙二胺四乙酸(EDTA,纯度99%) (物质的量比例为1∶1)加入烧瓶,添加30 mL水,90℃油浴搅拌6 h,取出倒入100 mL烧杯中,放入烘箱在80℃干燥12 h,然后在KTL-1400型高温管式炉中Ar气氛下以5℃/min 速率升温至600℃,热解2 h。热解得到的黑色固体用研钵磨碎,然后取过量的4 mol/L HCl酸洗12 h,过滤水洗,60℃干燥,得到Ni@NG。
1.2 Pt/Ni@NG催化剂制备
通过调变Pt的负载量和制备方法分别制备了Pt1/Ni@NG、Ptn/Ni@NG和Ptp/Ni@NG。Ni@NG负载的Pt单原子催化剂(Pt1/Ni@NG)采用浸渍法合成;Ni@NG负载的Pt团簇催化剂(Ptn/Ni@NG)和Pt纳米颗粒催化剂(Ptp/Ni@NG)采用沉积-沉淀法合成。采用浸渍法合成Pt1/Ni@NG需要将一定量的H2PtCl6·6H2O溶液(浓度为20 mg/mL)加入到2 mL乙醇中,然后加入200 mg的Ni@NG载体并在室温下搅拌20 h,随后把搅拌后的液体转移到60℃的真空干燥箱中烘干12 h得到固体粉末,粉末经过H2 200℃还原后得到Pt1/Ni@NG催化剂。采用沉积-沉淀法合成Ptn/Ni@NG和Ptp/Ni@NG需要将一定量的H2PtCl6·6H2O溶液加入到装有200 mg Ni@NG载体的圆底烧瓶中,将含有氯铂酸和载体的圆底烧瓶放入100℃油浴锅中加热1 h后取出,经过抽滤、洗涤、干燥后得到固体粉末,粉末经过H2 200℃还原后分别得到Ptn/Ni@NG和Ptp/Ni@NG催化剂。3种催化剂Pt理论负载量(质量分数)分别为0.1%、0.3%和0.5%,通过Agilent 5110电感耦合等离子发射光谱(ICP-OES)测得的实际负载量分别为0.1%、0.29%和0.53%。不同负载量的Pt/Ni@NG催化剂用Pt的理论质量分数表示,例如0.1%Pt/Ni@NG表示每100 mg催化剂中含有Pt元素的质量为0.1 mg。
1.3 硝基苯加氢
硝基苯加氢反应在NSC经典型快开式磁力搅拌反应釜中进行。通常情况下,在反应釜内衬中加入1 mmol硝基苯(纯度为99%)、10 mg Pt/Ni@NG催化剂、无水乙醇溶剂和1 mmol内标物苯甲醚(CP),总体积为10 mL。接下来,用高纯Ar气置换反应釜内空气3次,然后用磁力搅拌器边搅拌边加热到30℃,搅拌转速为800 r/min。当温度达到30℃并保持稳定后,引入H2并保持反应体系压力为1 MPa时开始进行硝基苯加氢反应,反应后用Agilent 7890A气相色谱对产物进行分析。基于ICP-OES分析测定了催化剂中Pt的实际负载量。转换频率(TOF)在保持底物转化率低于20%的情况下测定。硝基苯加氢反应的循环性能测试与前文的测试条件类似,在反应结束后,用磁铁收集催化剂,再用无水乙醇和水交替洗涤5次后放入烘箱干燥。之后在相同条件下进行测试,这样的过程重复5次。
TOF的计算公式如下[12]:
式中,n0为硝基苯初始进料摩尔数;c为硝基苯的转化率;t为反应时间;D为Pt分散度;ncat为催化剂中Pt的摩尔数。
1.4 结构表征
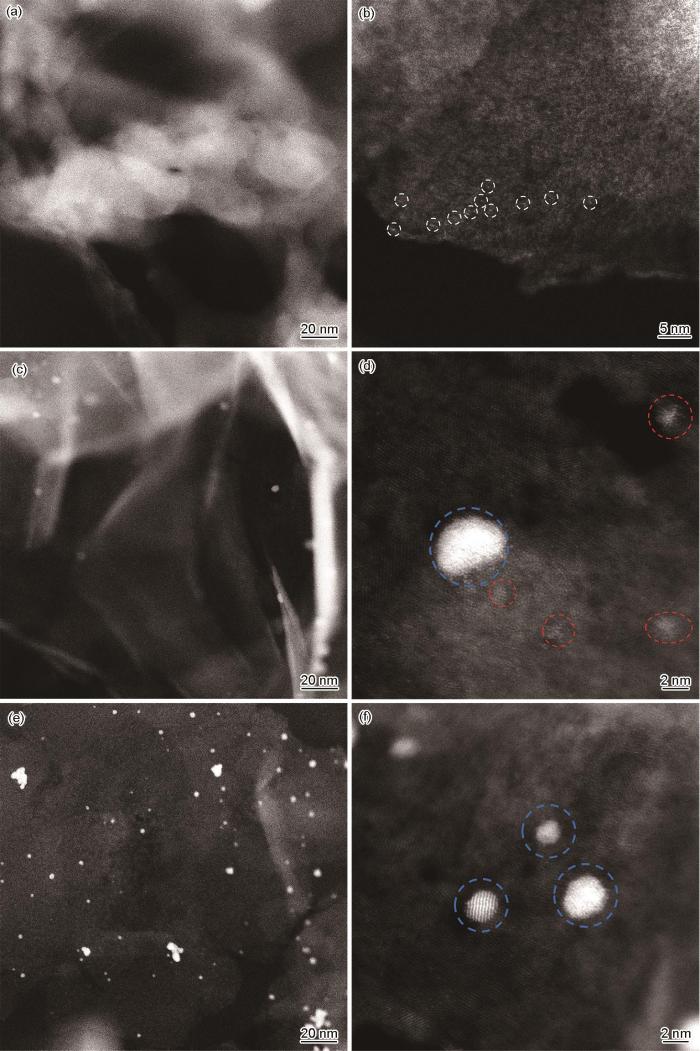
利用Tecnai G2 F20透射电子显微镜(TEM)观察Ni@NG载体结构特征;利用JEM ARM 200CF冷场发射球差校正透射电镜观察Pt/Ni@NG催化剂上Pt的分散情况;利用D8 Advance X射线衍射仪(XRD)分析多相材料Ni@NG的物相组成;利用EscaLab 250Xi X射线光电子能谱仪(XPS)分析Ni@NG载体表面信息。利用LabRam HR 800型Raman光谱仪表征Ni@NG载体表面石墨烯结构;利用ASAP 3020物理吸脱附仪测试Ni@NG载体表面孔径尺寸和比表面积;利用STA449F3热分析仪测试Ni@NG载体中Ni的成分含量。
2 实验结果与分析
2.1 Ni@NG载体的结构表征
图1
图1
Ni@NG载体的TEM分析和Ni纳米颗粒在Ni@NG载体中的尺寸分布
Fig.1
HRTEM images of Ni@NG support with different magnifications (a-d), high angle annular dark field (HAADF) STEM image of Ni@NG support (e), and size distributions of Ni nanoparticles in Ni@NG support (f) (Inset in Fig.1d shows the selected area electron diffraction pattern, d—lattice spacing)
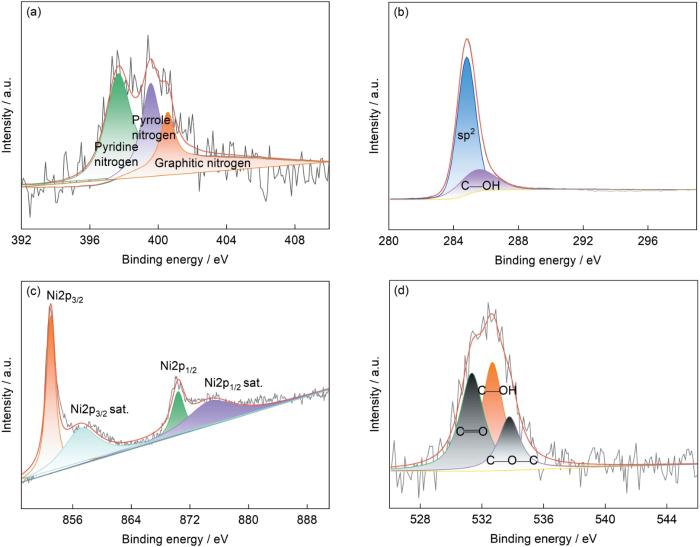
图2为制备的Ni@NG 载体的XPS结果。可以看出,N分别以吡啶氮、吡咯氮和石墨氮的形态分布在Ni@NG载体表面的石墨层上,其中吡啶氮与邻近的C原子组成的路易斯酸碱对可以促进硝基苯的吸附活化。Ni@NG表面基团还含有羰基、羟基和少量羧基,如图2b和d所示,对Ni元素的2p区域精细谱分析可知,2p3/2和2p1/2对应的峰位分别是852.9和870.2 eV,属于金属态Ni;其余几个峰位于858.2、874.3和881.2 eV,均为Ni的伴峰[11,20,21]。此外,针对酸洗后Ni@NG载体表面的XPS分析结果表明,石墨烯层上含有3.64%N、2.97%Ni、2.35%O和91.04%C (原子分数)。
图2
图2
Ni@NG载体的XPS表征结果
Fig.2
XPS results of the Ni@NG support
(a) N1s (b) C1s (c) Ni2p (d) O1s
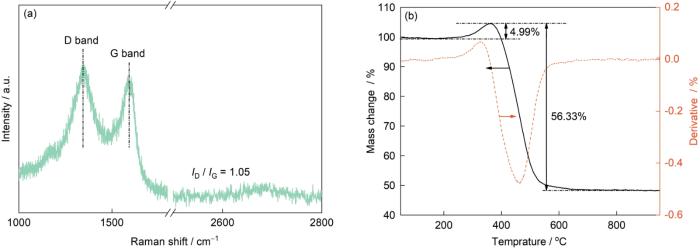
图3a为Ni@NG载体的Raman光谱。1341和1604 cm-1分别归属于石墨烯的D带和G带,其中D带由sp2 C原子间的对称伸缩振动引起,G带由sp2 C原子间的拉伸振动引起[22]。强度比ID / IG常用来表示石墨烯表面的无序排列程度,其值越高代表石墨烯表面无序程度越高,说明它的表面可能有更多缺陷。本工作制备的Ni@NG 载体的ID / IG 为1.05,说明石墨烯载体表面具有一定的C缺陷。此外在图3a中没有观察到Ni的特征峰,表明Ni在载体表面负载量很低,这与XPS的结果是吻合的。Ni@NG载体热重(TG)曲线如图3b所示。温度升高到400℃时,位于核中的Ni颗粒被氧化生成NiO,因此Ni@NG样品质量增加4.99%,随着温度进一步上升,C在高温空气中被消除,460℃时样品失重速率达到最大值,在600℃以后达到稳定状态,样品失重56.33%。Ni@NG样品剩余质量分数为48.66%,即Ni含量为 38.23%。
图3
图3
Ni@NG载体的Raman光谱和热重(TG)曲线
Fig.3
Raman spectrum (a) and thermogravimetric (TG) curves (b) of the as-prepared Ni@NG support (ID / IG—intensity ratio)
图4
图4
Ni@NG载体在加氢反应体系中的磁可分示意图
Fig.4
Schematics of the Ni@NG support with magnetic separability under the hydrogenation system
(a) the as-prepared Ni@NG sample was dispersed into the solvent
(b) then a magnet is placed on one side for 1 min
2.2 Pt/Ni@NG催化剂的表征
图5
图5
0.1%Pt1/Ni@NG、0.3%Ptn/Ni@NG和0.5%Ptp/Ni@NG催化剂的高角环形暗场(HAADF) STEM像
Fig.5
Low (a, c, e) and high (b, d, f) magnified HAADF STEM images of 0.1%Pt1/Ni@NG (a, b), 0.3%Ptn/Ni@NG (c, d), and 0.5%Ptp/Ni@NG (e, f) catalysts (White labels indicate Pt single atoms, blue labels indicate Pt nanoparticles, and red labels indicate Pt clusters)
图6
图6
Ni@NG载体和0.1%Pt1/Ni@NG、0.3%Ptn/Ni@NG、0.5%Ptp/Ni@NG催化剂的XRD谱
Fig.6
XRD spectra of Ni@NG support and 0.1%Pt1/Ni@NG, 0.3%Ptn/Ni@NG, and 0.5%Ptp/Ni@NG catalysts
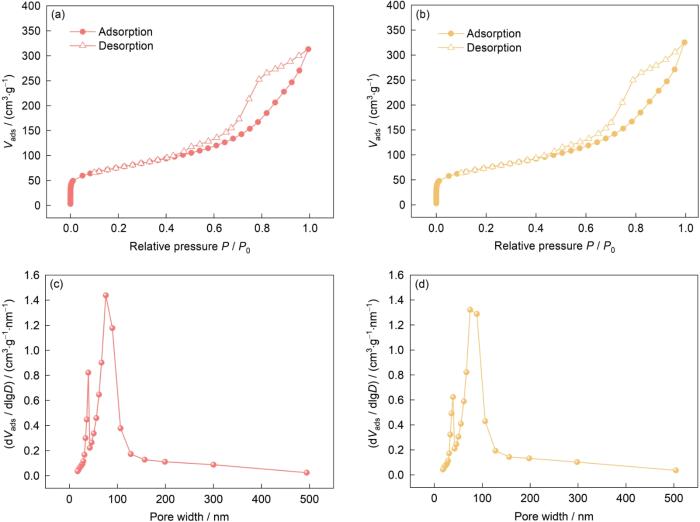
图7
图7
Ni@NG载体和0.3%Ptn/Ni@NG催化剂的N2吸脱附曲线和孔径分布曲线
Fig.7
N2 adsorption/desorption isotherms (a, b) and pore size distribution curves (c, d) of Ni@NG support (a, c) and 0.3%Ptn/Ni@NG catalyst (b, d) (Vads—N2 adsorption volume, D—pore size)
2.3 Pt/Ni@NG催化硝基苯加氢性能
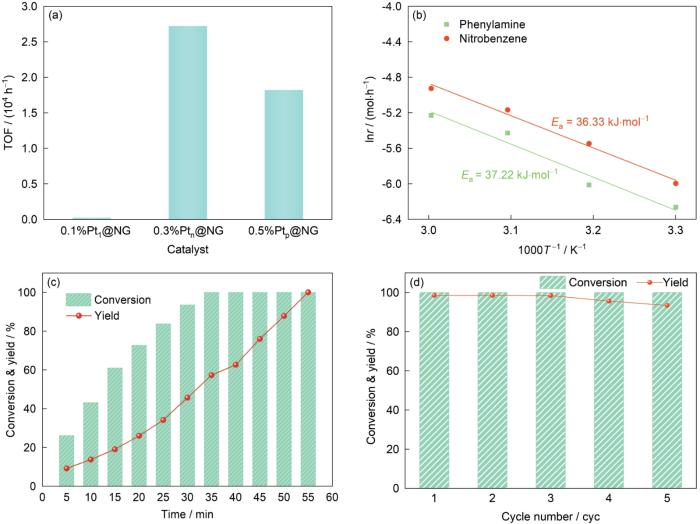
图8a显示了0.1%Pt1/Ni@NG、0.3%Ptn/Ni@NG和0.5%Ptp/Ni@NG催化剂上硝基苯加氢的TOF值。可见,0.3%Ptn/Ni@NG的TOF最高,为27239.2 h-1,而0.1%Pt1/Ni@NG和0.5%Ptp/Ni@NG催化剂的TOF分别为235.7和18225.3 h-1,表明在相同的反应条件下,0.3%Ptn/Ni@NG表现出最高的催化硝基苯加氢活性。如图5所示,0.3%Ptn/Ni@NG催化剂中Pt主要以团簇的形式和少量纳米颗粒的形式分散在Ni@NG载体上,0.5%Ptp/Ni@NG催化剂中Pt主要以纳米颗粒的形式分散在Ni@NG载体上,0.1%Pt1/Ni@NG催化剂上Pt主要以单原子的形式分散在Ni@NG载体的表面。已有研究[24]指出,当Pt负载在惰性载体Al2O3载体上,提高硝基苯的加氢活性和选择性需要适当的Pt物种聚集状态(3~5 nm),Pt物种在载体上团聚成颗粒会导致活性略微提升,但选择性急剧下降,当Pt物种均匀地分散到惰性载体Al2O3上形成单原子则完全没有加氢活性。根据硝基苯加氢机理[25],认为当Pt在Ni@NG载体上以团簇的形式存在更有利于硝基苯和H2的共同吸附活化,进而表现出最优的硝基苯加氢性能。
图8
图8
0.1%Pt1/Ni@NG、0.3%Ptn/Ni@NG和0.5%Ptp/Ni@NG催化剂上硝基苯加氢的转换频率(TOF),0.3%Ptn/Ni@NG催化剂的硝基苯加氢动力学曲线、硝基苯转化率和苯胺产率随时间变化曲线及硝基苯加氢循环稳定性
Fig.8
Turnover frequency (TOF) for 0.1%Pt1/Ni@NG, 0.3%Ptn/Ni@NG, and 0.5%Ptp/Ni@NG catalysts (a); nitrobenzene hydrogenation kinetic curves (b), time-nitrobenzene conversion rates and aniline yield curve (c), and hydrogenation stability (d) of 0.3%Ptn/Ni@NG catalyst (T—absolute temperature; r—reaction rate constant at temperature T; Ea—experimental activation energy)
基于0.3%Ptn/Ni@NG催化剂的优异性能,对其进行硝基苯催化加氢动力学测试,结果如图8b所示。可见,硝基苯加氢的活化能为36.33 kJ/mol,完全加氢到苯胺的活化能为37.22 kJ/mol,这表明在0.3%Ptn/Ni@NG催化剂上硝基苯不完全加氢的产物(如羟基苯胺、亚硝基苯)很容易被完全加氢生成苯胺[25~27]。图8c是0.3%Ptn/Ni@NG催化剂上硝基苯的转化率和苯胺的产率随时间的变化曲线。可见,随着时间的延长,硝基苯首先半加氢成羟基苯胺、亚硝基苯等中间产物,然后在60 min内完全转化成苯胺。此外,经过5 cyc反应后,0.3%Ptn/Ni@NG催化剂上苯胺的产率仅略微下降(图8d),表明催化剂具有优异的循环使用能力,这主要是由于Ni@NG载体自身的磁可分特性可以高效、快速地将0.3%Ptn/Ni@NG催化剂从液相反应体系分离,降低催化剂分离过程中造成的质量损失。本工作研究结果为开发低成本、环保、可重复使用的磁可分液相加氢催化剂提供了新的思路。
3 结论
本工作制备了一种C包覆Ni磁性载体负载Pt金属催化剂(Pt/Ni@NG),并将其应用于液相硝基苯加氢反应。以乙二胺四乙酸为C源和N源,Ni(OH)2为原料,经过煅烧碳化后即可制备具有磁可分性能的N掺杂石墨烯包覆Ni颗粒载体(Ni@NG)。将Pt/Ni@NG催化剂用于催化硝基苯加氢还原制苯胺,通过调控Pt的负载量和制备方法,在Ni@NG表面可控制备Pt单原子(0.1%Pt1/Ni@NG)、Pt团簇(0.3% Ptn/Ni@NG)、Pt纳米颗粒(0.5%Ptp/Ni@NG)催化剂。其中以Pt团簇结构为主的0.3%Ptn/Ni@NG催化剂表现出最优异的硝基苯加氢性能(TOF为27239.2 h-1)。这是因为相较于Pt单原子和Pt纳米颗粒,Pt团簇更有助于硝基苯和H2的吸附活化。此外,0.3%Ptn/Ni@NG催化剂的磁可分特性可以高效、快速地将催化剂从液相反应体系分离,保证催化剂的循环加氢稳定性。
参考文献
Transforming nano metal nonselective particulates into chemoselective catalysts for hydrogenation of substituted nitrobenzenes
[J].
Bio-waste chitosan-derived N-doped CNT-supported Ni nanoparticles for selective hydrogenation of nitroarenes
[J].In this study, a facile method for the synthesis of leach proof and earth-abundant non-noble Ni nanoparticles on N-doped carbon nanotubes is reported. The catalyst was synthesized by an impregnation-carbonization method, wherein a Ni-chitosan complex upon carbonization in a 5% H2/N2 atmosphere at 800 °C yielded Ni-containing N-doped CNTs. Chitosan served as a single source of carbon and nitrogen, and the nanotube growth was facilitated by the in situ formed Ni nanoparticles. The nanocatalyst was thoroughly characterized by several techniques; elemental mapping by SEM and TEM analysis confirmed the uniform distribution of Ni nanoparticles on the surface of N-doped CNTs with an average size in the range of 10-15 nm. The catalyst efficiently reduced a variety of nitroarenes (>99%) into their corresponding amines at a moderate pressure (5 bar) and a comparatively lower temperature (80 °C). Furthermore, the easy recovery of the catalyst using an external magnetic field along with high activity and easy recyclability makes the protocol eco-friendly.
Selective hydrogenation of nitrobenzene to aniline in dense phase carbon dioxide over Ni/γ-Al2O3: Significance of molecular interactions
[J].
Strong electronic interaction of indium oxide with palladium single atoms induced by quenching toward enhanced hydrogenation of nitrobenzene
[J].
Synergistic effect of acid-base and redox properties of nano Au/CeO2-cube on selective hydrogenation of nitrobenzene to aniline
[J].
Amine functionalization leads to enhanced performance for nickel- and cobalt-ferrite-supported palladium catalysts in nitrobenzene hydrogenation
[J].
Magnetically recoverable nanocatalysts
[J].
Toehold-containing three-way junction-initiated multiple exponential amplification and CRISPR/Cas14a assistant magnetic separation enhanced visual detection of Mycobacterium tuberculosis
[J].
Ionic liquid-functionalized magnetic metal-organic framework nanocomposites for efficient extraction and sensitive detection of fluoroquinolone antibiotics in environmental water
[J].
Magneto-responsive microneedle robots for intestinal macromolecule delivery
[J].
Nanoconfined nickel@carbon core-shell cocatalyst promoting highly efficient visible-light photocatalytic H2 production
[J].
A magnetically separable Pd single-atom catalyst for efficient selective hydrogenation of phenylacetylene
[J].
Earth-abundant Co nanoparticles encapsulated in N-doped hollow carbon sphere for highly selective hydrodeoxygenation of biomass-derived vanillin
[J].
Double-layer hybrid chainmail catalyst for high-performance hydrogen evolution
[J].
Interface synergism and engineering of Pd/Co@N-C for direct ethanol fuel cells
[J].Direct ethanol fuel cells have been widely investigated as nontoxic and low-corrosive energy conversion devices with high energy and power densities. It is still challenging to develop high-activity and durable catalysts for a complete ethanol oxidation reaction on the anode and accelerated oxygen reduction reaction on the cathode. The materials' physics and chemistry at the catalytic interface play a vital role in determining the overall performance of the catalysts. Herein, we propose a Pd/Co@N-C catalyst that can be used as a model system to study the synergism and engineering at the solid-solid interface. Particularly, the transformation of amorphous carbon to highly graphitic carbon promoted by cobalt nanoparticles helps achieve the spatial confinement effect, which prevents structural degradation of the catalysts. The strong catalyst-support and electronic effects at the interface between palladium and Co@N-C endow the electron-deficient state of palladium, which enhances the electron transfer and improved activity/durability. The Pd/Co@N-C delivers a maximum power density of 438 mW cm in direct ethanol fuel cells and can be operated stably for more than 1000 hours. This work presents a strategy for the ingenious catalyst structural design that will promote the development of fuel cells and other sustainable energy-related technologies.© 2023. The Author(s).
Distance synergy of MoS2-confined rhodium atoms for highly efficient hydrogen evolution
[J].
Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction
[J].Chainmail for catalysts: a catalyst with iron nanoparticles confined inside pea-pod-like carbon nanotubes exhibits a high activity and remarkable stability as a cathode catalyst in polymer electrolyte membrane fuel cells (PEMFC), even in presence of SO(2). The approach offers a new route to electro- and heterogeneous catalysts for harsh conditions.Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Ambient hydrogenation and deuteration of alkenes using a nanostructured Ni-core-shell catalyst
[J].
Activity promotion of core and shell in multifunctional core-shell Co2P@NC electrocatalyst by secondary metal doping for water electrolysis and Zn-air batteries
[J].
Supported Ni0@C-N catalyst with dual-reaction surfaces: Structure-performance relation in the selective hydrogenation of p-chloronitrobenzene
[J].
N-doped core-shell mesoporous carbon spheres embedded by Ni nanoparticles for CO2 electroreduction
[J].
Magnetically induced catalytic reduction of biomass-derived oxygenated compounds in water
[J].The development of energetically efficient processes for the aqueous reduction of biomass-derived compounds into chemicals is key for the optimal transformation of biomass. Herein we report an early example of the reduction of biomass-derived oxygenated compounds in water by magnetically induced catalysis. Non-coated and carbon-coated core-shell magnetic nanoparticles were used as the heating agent and the catalyst simultaneously. In this way it was possible to control the product distribution by adjusting the field amplitude applied during the magnetic catalysis, opening a precedent for this type of catalysis. Finally, the encapsulation of the magnetic nanoparticles in carbon () strongly improved the stability of the magnetic catalyst in solution, making its reuse possible up to at least eight times in dioxane and four times in water.© 2022 American Chemical Society.
Structure sensitivity of acetylene semi-hydrogenation on Pt single atoms and subnanometer clusters
[J].
Appropriate aggregation is needed for highly active Pt/Al2O3 to enable hydrogenation of chlorinated nitrobenzene
[J].
Theoretical study on nitrobenzene hydrogenation by N-doped carbon-supported late transition metal single-atom catalysts
[J].
Highly efficient hydrogenation of nitrobenzene to aniline over Pt/CeO2 catalysts: The shape effect of the support and key role of additional Ce3+ sites
[J].
Modulating the catalytic behavior of non-noble metal nanoparticles by inter-particle interaction for chemoselective hydrogenation of nitroarenes into corresponding azoxy or azo compounds
[J].