不锈钢是临床常用的金属材料,可作为心血管支架、正畸托槽等医疗器械,应用于心脑血管治疗以及口腔修复[1~4]。奥氏体不锈钢由于其优异的力学性能与耐腐蚀性能在医疗领域得到了广泛应用[5~9]。但在实际使用时,致病微生物易在材料表面增殖并引起细菌感染等问题[10]。细菌通过分泌蛋白质、多糖等生物大分子形成胞外聚合物[11,12],并在其黏附和包裹下,以附着力较强的生物膜形式附在材料表面。细胞间生物膜的群体感应能够刺激基因表达,大大提高了病原体在恶劣条件下的生存能力[13~15],从而容易引起材料周围的人体组织发炎,并导致材料服役性能失效。因此,赋予不锈钢良好的抗菌性能是降低细菌感染风险的可行方案[12,16,17]。人们使用不同方法制备抗菌不锈钢,包括表面改性、表面涂层、合金化方式以及复合制备方法。其中合金化方式是在不锈钢中加入具有抗菌性能的金属元素,经过特殊热处理制备成合金型抗菌不锈钢[16,17],能够保证不锈钢自身力学性能的同时实现广谱抗菌效果。
在诸多抗菌元素中,Ga元素近年来被证实具有良好的抗菌性能。早期,镓化合物作为抗癌与抗肿瘤药物被广泛应用于医疗卫生领域[18~23]。以柠檬酸缓冲的Ga(NO3)3等已被美国食品药物管理局(FDA)批准应用于抗癌等相关医学药物治疗中[24]。同时,Ga有助于抑制骨髓瘤和骨转移,具有治疗骨质疏松的潜力[18,25]。随着人们对Ga的抗菌研究逐渐深入,发现以镓化合物为主的镓制剂在体内外均有明显的抑菌作用[26~31]。如Ga(NO3)3[26~28]和麦芽酸镓[29,30]对革兰氏阴性菌(如铜绿假单胞菌[26]、大肠杆菌[31])和革兰氏阳性菌(如金黄色葡萄球菌[27,30]、表皮葡萄球菌[30])均具备有效的灭活作用。Ga能够抑制生物膜的形成,破坏生物膜基质中的细菌细胞[32,33]。Ga甚至具有抗病毒活性,可作为COVID-19的潜在治疗方法[34]。此外,Ga具有较好的生物相容性,无细胞毒性[35]。
已有研究[18,36]表明,Ga3+的抗菌效果取决于Ga与Fe物理化学性质的相似性。Ga易与细菌携带铁载体结合,影响细菌的铁吸收和代谢,进而导致细菌死亡[24,31]。Ga被细菌吸入体内后,降低胞内相关酶活性,并且破坏电子传递链,在体内产生活性氧(ROS)并引起细菌的氧化应激反应[24,31,37~40]。Ga在生理条件下极易水解,因此目前的研究多集中在含Ga化合物中的抗菌性[24],而对镓合金的抗菌研究相对较少。Li等[37]发现Ga在共晶GaIn合金中为单质状态,具有广谱杀菌活性,并且与同浓度KNO3溶液相比,GaIn合金的抑菌效果更为显著。Cochis等[38]发现Ga在钛合金中具有抗菌活性,其抗菌性能与合金中Ga含量以及合金与细菌的共培养时间有关。此外,Ti-Ga合金能显著降低生物膜的数量和活性,具有良好的细胞相容性[38]。同时,研究[41]发现FeGa合金具有良好的生物相容性,在生物医学领域具有广阔的应用前景。因此,Ga的合金化是一种可行的抗菌策略。
基于上述背景,本工作拟在304L奥氏体不锈钢中通过冶金制备手段添加金属Ga,探究Ga添加对304L不锈钢力学性能与抗菌性能的影响,并初步阐明Ga在不锈钢合金化中的抗菌机理。根据Fe-Ga二元相图可知,Ga是铁素体稳定元素,3.5%Ga (质量分数)可完全溶解在γ-Fe中,初步证明了Ga添加的可行性。
1 实验方法
1.1 实验材料、溶液及细菌培养
304L及304L-Ga不锈钢均在5 kg真空感应炉中炼制,具体成分如表1所示。铸锭经锻造后在1100℃下热轧成3 mm厚的板材。试样经过空冷处理后放入真空封管中,在1050℃下进行30 min的固溶处理,水冷至室温。
表1 304L及304L-Ga不锈钢试样的化学成分 (mass fraction / %)
Table 1
| Steel | C | P | S | Si | Mn | Ni | Cr | Ga | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 304L SS | 0.01 | 0.01 | 0.02 | 0.48 | 0.40 | 7.89 | 17.21 | - | Bal. |
| 304L-Ga SS | 0.02 | 0.02 | 0.02 | 0.70 | 0.96 | 8.03 | 17.95 | 1.57 | Bal. |
实验所用细菌分别为革兰氏阴性菌大肠杆菌(E. coli,ATCC 25922)和革兰氏阳性菌变形链球菌(S. mutans,ATCC 25175)。E.coli采用的LB (Luria-Bertani)培养基每升的组分为:牛肉膏5 g,NaCl 5 g,蛋白胨10 g。相应的固体培养基中加入20 g琼脂。用4%NaOH (质量分数)调节培养基pH = 7.2 ± 0.1。S. mutans采用的BHI (brian heart infusion)固体培养基每升的组分为:10 g胰蛋白胨,17.5 g牛心浸出粉,5 g NaCl,2 g葡萄糖,2.5 g Na2HPO4,pH = 7.4 ± 0.2,外加20 g琼脂。变形链球菌培育时需放在厌氧罐中,在标准厌氧条件下(80%N2 + 10%H2 + 10%CO2,37℃)进行繁育。
浸泡实验所用的测试溶液为模拟唾液,每升的成分为:0.4 g NaCl,0.4 g KCl,0.69 g NaH2PO4·2H2O,0.684 g CaCl2,0.005 g Na2S·9H2O,1 g尿素。
1.2 实验方法
沿板材轧制方向切取样品,采用Inspect F50 扫描电镜(SEM)和能谱仪(EDS)对抛光和电解腐蚀(电压1.5~2 V,电流约50 mA,电解1~2 min)后的样品进行显微组织和成分分析,采用D8 Advance X射线衍射仪(XRD)测定样品的相组成并计算不锈钢晶格常数,CuKα,扫描角度40°~102°,扫描速率1.2°/min。使用Instron万能试验机测试含Ga不锈钢的室温拉伸性能,拉伸速率为1 mm/min。试样沿板材轧制方向按图1所示尺寸取样,厚度为3 mm。每种不锈钢准备3组拉伸试样作为平行样。使用HV-1000型数显硬度仪测试含Ga不锈钢的Vickers硬度(施加载荷9.8 N,加载时间10 s),对每个试样测量5个点,取其平均值。
图1
使用平板计数法评价材料对细菌的抗菌性能。实验前,将抛光后的样品用75%乙醇清洗10 min,用紫外线照射消毒15 min,放入24孔板中。使用磷酸盐缓冲溶液(PBS)将E. coli与S. mutans的浓度分别稀释到105和106 CFU/mL。取每种细菌菌悬液各50 μL分别滴于样品表面,在(37 + 2)℃下共培养24 h。共培养后,将样品表面E. coli和S. mutans的浓度稀释至104 CFU/mL,取100 L悬浮液分别滴入固体LB培养基和BHI培养基中并均匀摊开。将带有2种细菌的培养基分别在需氧条件和厌氧条件下培养,24 h后分别计数菌落数,使用如下公式计算304L-Ga不锈钢的抗菌率(K):
式中,NC和NS分别为对照组(304L)和实验组(304L-Ga)的菌落计数。使用Inspect F50 SEM观察样品表面细菌形态。细菌与样品共培养24 h后取出,PBS溶液仔细清洗样品表面后,使用2.5% (体积分数)戊二醛在4℃下固定4 h,然后用不同浓度(50%、60%、70%、80%、90%、95%、100%)的乙醇溶液进行逐级脱水(每级10 min)。样品在室温下干燥后做喷金处理,在20 kV高真空模式下用Inspect F50 SEM观察。
使用ROS检测试剂盒检测细菌细胞内的ROS表达情况。细菌与样品共培养24 h。之后,将1 mL 10 μmol/L浓度的荧光探针DCFH-DA滴加到样品上,与样品在37℃下培养30 min,使用BX53+DP74荧光显微镜观察处理后细菌的荧光产物2',7'-二氯荧光素(DCF)。脂质过氧化是ROS对细菌造成的一种典型损伤,细菌膜中的不饱和脂肪酸被ROS氧化生成降解产物丙二醛(MDA),产物随之参与一系列反应最终导致细菌死亡。作为脂质过氧化和代谢细胞损伤的代谢标志,MDA能够与硫代巴比妥酸(TBA)发生显色反应,形成的化合物在535 nm处有光密度(OD535)[42]。107 CFU/mL浓度的E. coli和S. mutans分别与样品共培养24 h后,将细菌在等体积的冰10%三氯乙酸溶液(TCA,10 g TCA加入到100 mL水中)中培养20 min,之后裂解液以约9500 r/min转速离心1 min。取1 mL上清液并滴入1 mL 0.6% TBA溶液(取0.6 g TBA粉末,加入1 mol/L NaOH溶液约20 mL,保证粉末充分溶解,使用10%TCA溶液定容至100 mL),在热水中煮沸2 h。冷却后,使用紫外分光计在535 nm处测定混合物中形成的MDA含量。使用PBS溶液配置108 CFU/mL浓度的2种细菌。根据ISO 10993-12:2002,将10 mL的2种细菌和PBS溶液分别与4个抛光的304L-Ga不锈钢样品(12.5 cm2)在37℃下浸泡24 h。使用iCAP Q电感耦合等离子体源质谱仪(ICP-MS)测试浸泡溶液中的Ga3+浓度。
使用ESCALAB 250型X射线光电子能谱仪(XPS)分析304L-Ga不锈钢在溶液中浸泡前后表面Ga的化学状态。准备2组样品,一组打磨后使用无水乙醇清洗吹干。一组样品在37℃的模拟唾液中浸泡24 h,浸泡后轻取并自然晾干。实验结果通过标准C谱(284.6 eV)校准,并使用XPS PEAK软件进行数据处理。
2 实验结果
2.1 含Ga不锈钢的显微组织
图2
图2
304L和304L-Ga不锈钢显微组织的SEM像以及Ga在304L-Ga不锈钢中的EDS分布
Fig.2
SEM images of 304L SS (a) and 304L-Ga SS (b), and EDS distribution of Ga in 304L-Ga SS (c)
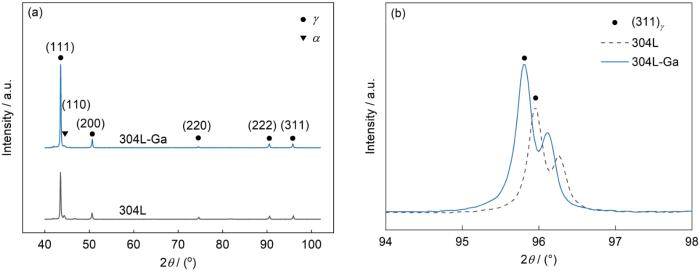
图3为304L和304L-Ga不锈钢的XRD谱。结果表明,304L和304L-Ga不锈钢均以奥氏体为主,存在极少量的铁素体。微量Ga添加没有引入新相形成,对铁素体的形成影响不大。图3b中304L-Ga不锈钢的高角度峰(311) γ 向左偏移,利用Bragg方程计算得到304L和304L-Ga不锈钢的晶格常数分别为0.3442和0.3446 nm,说明Ga的加入导致材料晶格常数增加。这是由于Ga原子半径(0.135 nm)略大于Fe原子半径(0.124 nm),因此Ga原子在钢中会取代Fe原子晶格位点,形成置换固溶体[31]。因此,Ga添加使得不锈钢的晶胞参数略有增大,晶面间距增大,导致衍射峰略微向左偏移。
图3
图3
304L和304L-Ga不锈钢的XRD谱和高角度峰衍射谱
Fig.3
XRD spectra of 304L SS and 304L-Ga SS (a) and detailed scans for top diffraction peak (b)
2.2 力学性能表征
图4
图4
304L和304L-Ga不锈钢的工程应变-应力曲线和力学性能
Fig.4
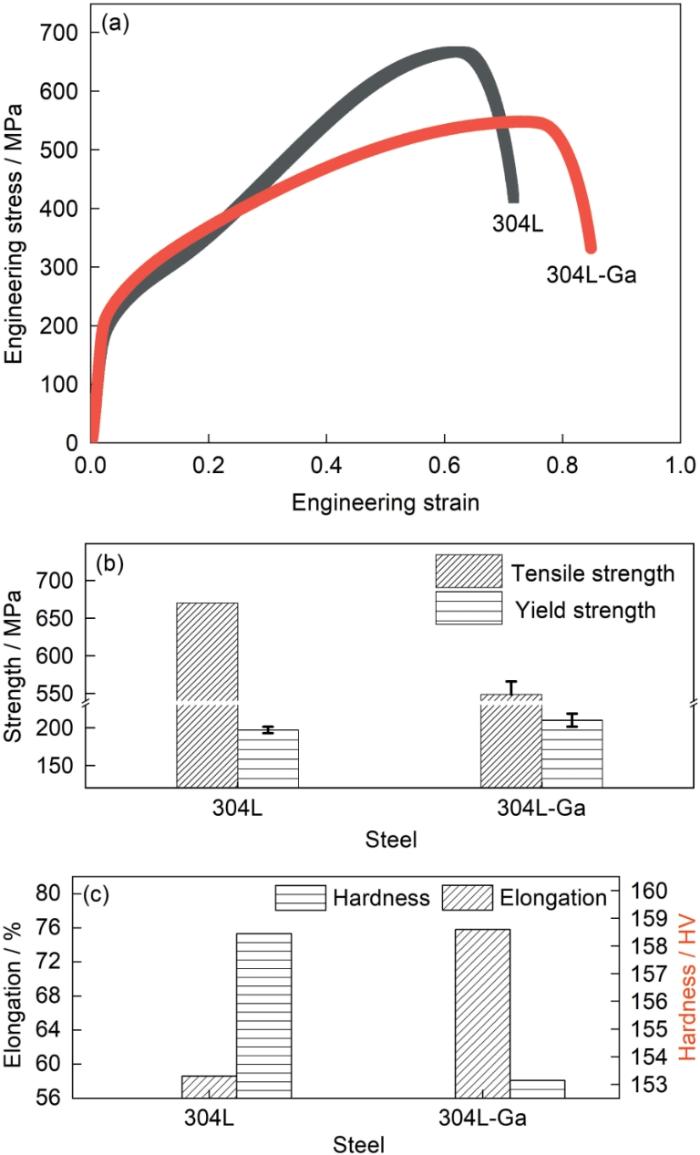
Engineering stress-strain curves (a), and varia-tions of tensile and yield strengthes (b) and elongation and hardness (c) of 304L SS and 304L-Ga SS
表2 304L和304L-Ga不锈钢力学相关参数
Table 2
| Steel | RmMPa | RelMPa | A% | Hardness HV |
|---|---|---|---|---|
| 304L SS | 670 | 197 ± 4.2 | 58.6 ± 1.3 | 158.45 ± 6.29 |
| 304L-Ga SS | 548.3 ± 17.6 | 210 ± 8.7 | 75.8 ± 3.3 | 153.14 ± 4.20 |
材料的形变本质是位错的迁移,材料强度与塑性的变化是内部位错运动状态改变的体现。屈服强度体现材料抵抗微量变形的能力。Ga原子在铁基材料内部以置换固溶体形式存在,在位错运动初期通过钉扎位错,提高材料的屈服强度。由于Ga的添加量较小,使得材料屈服强度小幅度增加。而材料的抗拉强度体现材料抵抗均匀塑性变形的能力,即对位错滑移的抵抗作用。越过屈服点后,材料塑性变形开始,材料内部位错大量增殖。此时,微量Ga原子难以对大量位错产生明显的钉扎作用。相反,Ga合金化对堆垛层错能的影响突显,使位错选择能量更低的方式运动。
堆垛层错能(SFE)决定堆垛层错发生的几率。合金SFE越高时,对应的扩展位错变窄。此时,位错在变形过程中更容易形成束集并发生交滑移,减少了位错塞积群的产生,使得不锈钢内产生堆垛层错的概率降低[43]。由于Ga原子的fcc的堆垛方式与铁基材料的ABAB式层错结构不同,因此Ga不容易嵌入到铁基材料的层错中,减少了新层错的产生,提高了堆垛层错能。层错能的增加促进了部分位错向另一滑移面滑移,防止了部分位错的堆积,使不锈钢的强度降低而伸长率增加。
此外,溶质原子和基体原子的电子结构不同可以调节材料的层错能[44]。Ga的外层电子结构(4s23d104p1)与Fe (3d64s2)不同,Ga的4p1电子进入到Fe的3d电子层时,会干扰Fe的对称点阵结构。并且Ga原子与Fe原子之间不能形成强化学键,Ga无法钉扎在层错中,导致层错能的增加。
因此可以认为,材料强度和延伸率的变化是层错能增加和固溶强化协同作用的结果。Ga添加通过增大层错能,减小堆垛层错出现的几率,使位错更容易产生交滑移,减少位错缠结和位错塞积,使得材料抗拉强度减小,同时塑性增强,表现为断后伸长率增大。
2.3 抗菌性能及生物膜观察
图5
图5
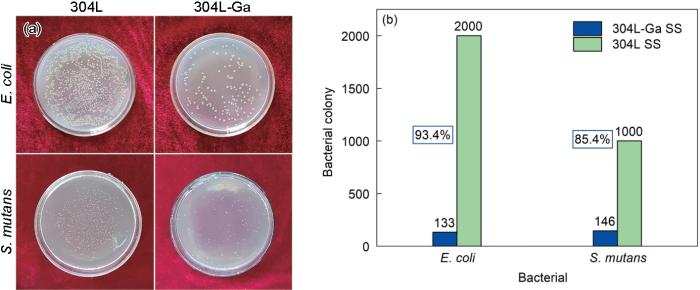
E. coli和S. mutans与304L和304L-Ga不锈钢共培养后生长的菌落情况及304L-Ga不锈钢的抗菌率
Fig.5
Bacterial colonies of E. coli and S. mutans after co-culture with 304L SS and 304L-Ga SS (a) and the antibacterial rates of 304L-Ga SS against E. coli and S. mutans (b)
细菌与不锈钢孵育24 h后在其表面的附着形态如图6所示。可以看出,304L不锈钢表面附着大量菌落,细菌细胞表面光滑,形状饱满。并且有明显团簇状菌落,说明形成了生物被膜。而304L-Ga不锈钢表面团簇状菌落逐渐消失,细菌基本以个体形式存在,并且较为分散。同时,细菌的细胞膜受到破坏,细菌形态由饱满状态变为坍缩状态,甚至发生裂解。说明含Ga不锈钢能够有效抗菌,影响细菌生长形态与细胞膜的活性,同时能够抑制不锈钢表面细菌生物被膜的形成。
图6
图6
E. coli和S. mutans在304L和304L-Ga不锈钢的菌落形态
Fig.6
SEM images of E. coli (a, b) and S. mutans (c, d) after co-culture with 304L SS (a, c) and 304L-Ga SS (b, d) for 24 h
2.4 ROS产物分析
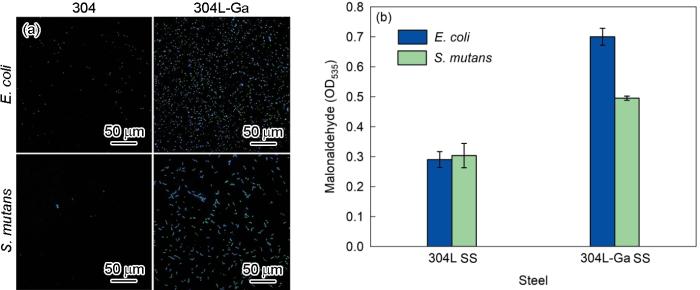
ROS由细菌产生,可导致细菌细胞的氧化应激,能够有效破坏细胞膜、蛋白质、脂质等有机体中的重要分子,最终导致机体氧化损伤甚至死亡[39]。图7为2种细菌与不锈钢共培养后的DCF荧光强度和MDA含量水平,分别代表细菌ROS产生水平和细胞的脂质过氧化程度。如图7a所示,与304L共培养的E. coli和S. mutans几乎没有产生ROS,荧光强度较弱。而与304L-Ga不锈钢共培养后,E. coli和S. mutans中的ROS水平显著升高,说明合金中Ga添加导致了细菌中ROS的高表达。脂质过氧化是细菌产生ROS的主要损伤形式之一[42]。如图7b所示,与304L相比,304L-Ga不锈钢表面的细菌产生的MDA含量明显增加,说明304L-Ga表面的细菌破碎更多,与上述ROS荧光结果一致。这表明,Ga添加导致细菌ROS高表达,破坏细菌细胞膜,导致细胞脂质过氧化,使得细菌死亡。因此,诱导细菌产生ROS并破坏细胞膜是含Ga不锈钢的抗菌机制之一。
图7
图7
E. coli和S. mutans在304L和304L-Ga不锈钢共培养24 h后的2',7'-二氯荧光素(DCF)荧光强度和丙二酫(MDA)生成含量
Fig.7
DCF fluorescence intensities (a) and MDA (b) of bacteria co-cultured with 304L SS and 304L-Ga SS at 37oC for 24 h (DCF—2',7'-dichlorofluorescein, MDA—malonadehyde, OD—optical density)
2.5 Ga3+ 溶出
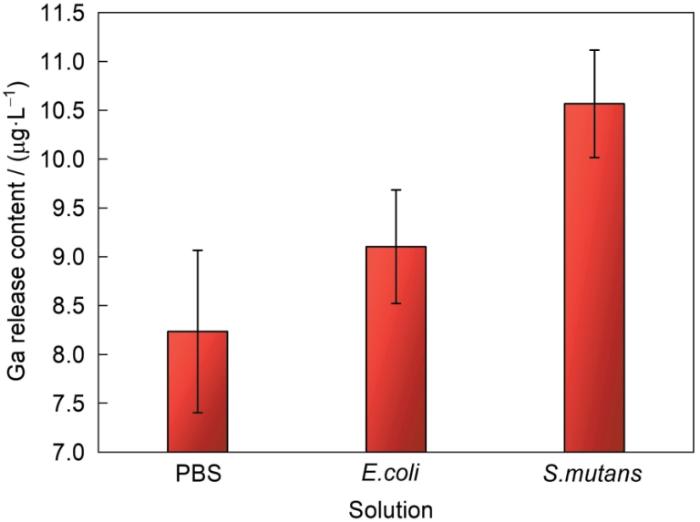
为了验证不锈钢的抗菌性是否来源于表面所析出的Ga3+,将304L-Ga不锈钢在含有2种细菌的溶液以及PBS缓冲液中分别浸泡24 h,检测溶液中释放的Ga3+浓度,结果如图8所示。可以看出,在3种溶液中均检测到微量的Ga3+,证明不锈钢表面能够向溶液中有效溶出Ga3+。与2种菌液相比,PBS中溶出的Ga3+含量明显减少,说明细菌与不锈钢的接触促进了样品表面Ga3+的释放。
图8
图8
304L-Ga不锈钢在37℃与不同溶液共培养24 h后析出的Ga3+浓度
Fig.8
Ga3+ concentrations in different solutions after co-cultured with 304L-Ga SS at 37oC for 24 h (PBS—phosphate buffer solution)
由于Ga3+在生理环境中极易水解并形成Ga(OH)3等化合物[45~47],会降低溶液中有效Ga3+的含量。因此,当Ga(NO3)3类化合物加入到溶液中时,Ga3+倾向于完全水解。Hijazi等[48]证实Ga(NO3)3对E. coli的最低抑制浓度(MIC)为8 μmol/L (139.4 μg/L),意味着需要更高浓度的Ga3+以抵消水解产生的消耗。图8中可知,304L-Ga不锈钢在E. coli溶液中浸泡24 h后所释放的Ga3+约为9 μg/L,而抑菌率达到90%以上。镓合金达到与镓化合物相同抗菌率时所溶出的Ga3+含量远远小于化合物需要的Ga3+浓度水平,说明合金中溶出的新鲜未水解的Ga3+具有更强的抗菌活性。由此推测,细菌在接触304L-Ga不锈钢后,将Ga3+在水解前瞬间捕获,减弱了Ga3+的水解反应。同时,与PBS相比,细菌与钢表面的接触促进了Ga3+的释放,导致菌液中Ga3+含量增加。
2.6 Ga在不锈钢中分布
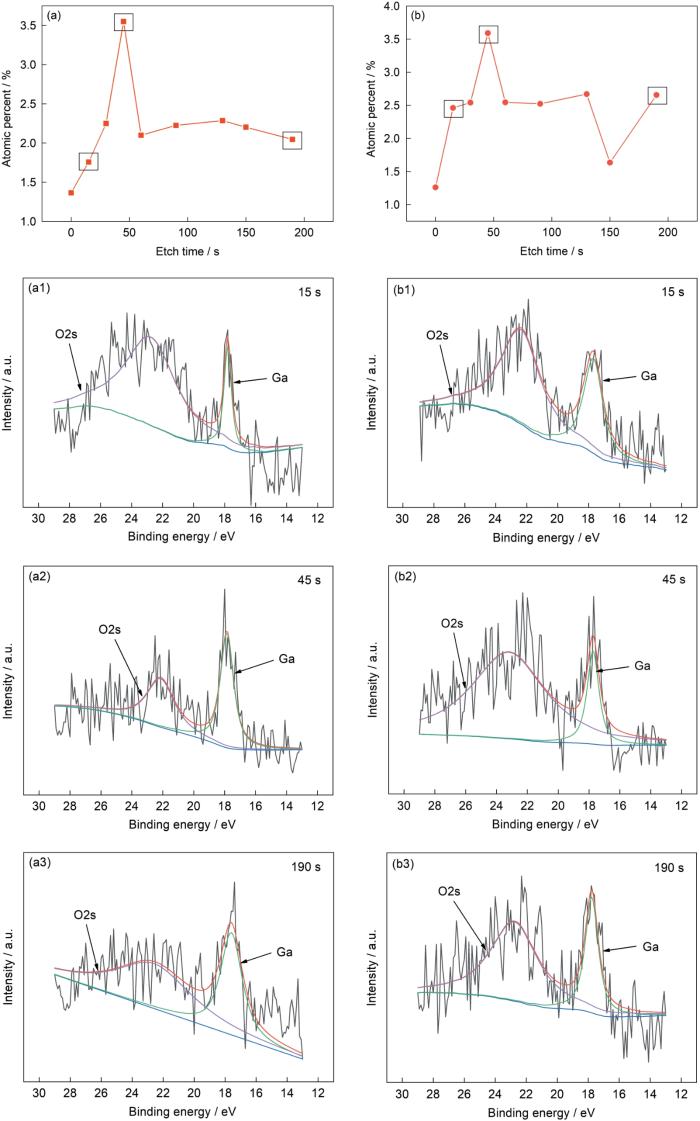
为分析溶液中Ga3+的溶出来源,并进一步研究不锈钢的抗菌机理,使用XPS分析钝化膜中的Ga在浸泡前后的化学状态变化。图9为浸泡前后304L-Ga不锈钢钝化膜中Ga浓度深度分布曲线和不同溅射深度下Ga3d精细谱的拟合曲线。如图9a所示,浸泡前不锈钢内Ga含量随溅射时间变化波动较大,说明Ga在深度方向上分布不均匀。当溅射到达钝化膜深处时,Ga含量达到峰值。而溅射逐渐进入基体时,Ga含量趋于稳定。进一步分析Ga在钝化膜以及基体中的化学价态和存在形式,根据Ga的浓度曲线选择3个特殊位置,分别代表不锈钢钝化膜表面(15 s)、钝化膜深度(45 s)和基体中(190 s)。图9a1~a3显示了3个深度下Ga3d精细谱拟合结果。可见,3个拟合谱中均只有单质Ga,对应峰位为17.8 eV,说明Ga在钝化膜和基体中以合金状态Ga存在,不形成氧化物。
图9
图9
304L-Ga不锈钢在模拟唾液中浸泡前后表面Ga的化学状态
Fig.9
Depth profiles of Ga concentrations on the surficial passivation film before (a) and after (b) soaking in the simulant saliva, and peak fitting curves of Ga3d spectrograms before soaking (a1-a3) and after soaking for different etch time (b1-b3)
2.7 抗菌机理分析
根据本工作结果并结合前人工作,得出了304L-Ga不锈钢的抗菌机理,如图10所示。
图10
图10
304L-Ga不锈钢的抗菌机理示意图
Fig.10
Schematic of antibacterial mechanism of 304L-Ga SS
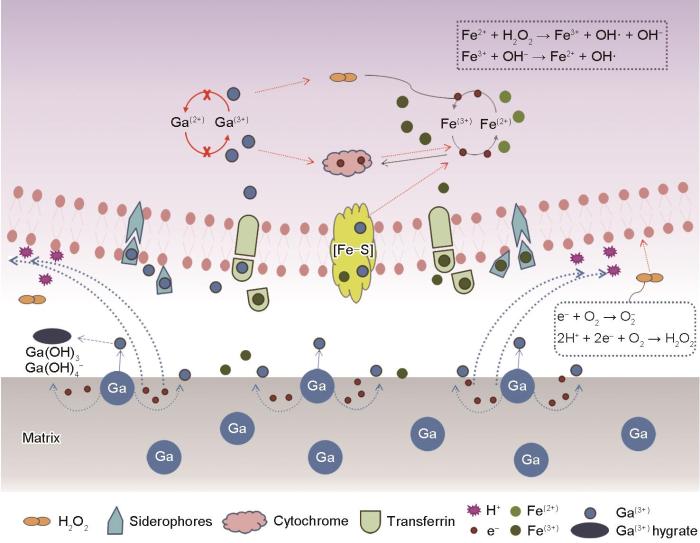
由于Ga和Fe的相似性,Ga3+被细菌错认作Fe3+而被吸入体内,与细胞内铁载体或相关蛋白结合,导致细菌无法完成Fe3+和Fe2+之间的转化,影响细胞内Fe3+的吸收和Fe2+的形成[19,49]。并且Ga3+不能转为其他价态,无法完成细胞内所需的Fe3+到Fe2+的价态转变,对应的氧化还原过程受到影响。另一方面,Ga破坏细菌的Fenton反应,导致细胞内ROS高表达,造成氧化损伤[50]。同时,Ga与细胞色素等电子传递相关蛋白结合,影响电子传递链,从而影响细菌代谢过程[39]。因此,Ga的摄入影响细胞内铁吸收与铁代谢,导致细菌吸收过量的Fe3+以满足细胞代谢,并且使细胞内ROS高表达,造成细胞损伤。
此外,与镓化合物相比,含Ga不锈钢所溶出的Ga3+浓度较低,但具有更好的抗菌性能。并且上文分析得出,相比于PBS溶液,细菌接触不锈钢后促进了Ga3+的析出。可以认为,与镓化合物相比,细菌与含Ga不锈钢表面的直接接触是含Ga不锈钢的另一抗菌机制。由于Ga与不锈钢的基体Fe原子相比具有更低的标准电极电位(
在此过程中反应释放的O2-、H2O2等ROS同样损伤细菌细胞膜,并诱导其最终死亡[52]。
综上,含Ga不锈钢的抗菌机理可简述如下:Fe与Ga的相似性使得Ga被吸入细菌,与铁载体等相关蛋白结合,影响铁吸收和铁代谢;Ga干扰胞内Fenton反应以及电子传递过程,诱导活性氧高表达,损伤细菌细胞膜;细菌与含Ga不锈钢的接触中Ga溶解,促进Ga3+释放,同时在接触过程产生的H2O2等ROS对细菌造成进一步损伤,诱导细菌死亡。
本实验中,与E. coli相比,S. mutans促进Ga3+释放的能力更强,而含Ga不锈钢对S. mutans的抗菌效果更弱。同时,S. mutans体内释放ROS含量与E. coli相比较少。这是由于不同细菌表面的负电荷影响抗菌结果。革兰氏阴性菌E. coli表面的负电荷较革兰氏阳性菌S. mutans多,对材料释放的电子的消耗较少,因此E. coli促进Ga3+溶出能力较弱,表现为E. coli菌液中测得离子浓度较小。S. mutans作为兼性厌氧菌,体内大量的超氧化物歧化酶(SOD酶)、过氧化氢酶等物质可以分解ROS,防御氧化自由基的损伤,缓解ROS的细胞毒性。
3 结论
(1) 添加Ga后,304L-Ga不锈钢仍为奥氏体组织,Ga在不锈钢内作为置换固溶体存在,起到固溶强化作用,提高了材料的屈服强度和伸长率,降低材料的抗拉强度和硬度。
(2) Ga在不锈钢钝化膜与基体中以合金形式存在,在溶液中浸泡时由Ga释放Ga3+。
(3) 304L-Ga不锈钢具有优异的抗菌性能。Ga3+通过引起细菌体内活性氧的高表达,诱导细菌的氧化应激,导致细菌细胞膜损伤死亡。同时细菌在接触不锈钢表面时瞬间捕获溶出的Ga3+,减小离子水解几率,同时捕获电子,促进Ga溶解与Ga3+的释放,并释放过量ROS进一步导致细菌死亡。
参考文献
A stainless steel bracket for orthodontic application
[J].
Review of materials in medical applications
[J].
The synergistic effect of proteins and reactive oxygen species on electrochemical behaviour of 316L stainless steel for biomedical applications
[J].
Research on the mechanical properties for medical stainless steel
[J].
Annealing of cold-worked austenitic stainless steels
[J].
In situ investigations of microstructural changes during tensile deformation of AISI 304L stainless steels
[J].
Antibacterial metals and alloys for potential biomedical implants
[J].Metals and alloys, including stainless steel, titanium and its alloys, cobalt alloys, and other metals and alloys have been widely used clinically as implant materials, but implant-related infection or inflammation is still one of the main causes of implantation failure. The bacterial infection or inflammation that seriously threatens human health has already become a worldwide complaint. Antibacterial metals and alloys recently have attracted wide attention for their long-term stable antibacterial ability, good mechanical properties and good biocompatibility and. In this review, common antibacterial alloying elements, antibacterial standards and testing methods were introduced. Recent developments in the design and manufacturing of antibacterial metal alloys containing various antibacterial agents were described in detail, including antibacterial stainless steel, antibacterial titanium alloy, antibacterial zinc and alloy, antibacterial magnesium and alloy, antibacterial cobalt alloy, and other antibacterial metals and alloys. Researches on the antibacterial properties, mechanical properties, corrosion resistance and biocompatibility of antibacterial metals and alloys have been summarized in detail for the first time. It is hoped that this review could help researchers understand the development of antibacterial alloys in a timely manner, thereby could promote the development of antibacterial metal alloys and the clinical application.© 2021 The Authors. Production and hosting by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Microbial biofilms
[J].Direct observations have clearly shown that biofilm bacteria predominate, numerically and metabolically, in virtually all nutrient-sufficient ecosystems. Therefore, these sessile organisms predominate in most of the environmental, industrial, and medical problems and processes of interest to microbiologists. If biofilm bacteria were simply planktonic cells that had adhered to a surface, this revelation would be unimportant, but they are demonstrably and profoundly different. We first noted that biofilm cells are at least 500 times more resistant to antibacterial agents. Now we have discovered that adhesion triggers the expression of a sigma factor that derepresses a large number of genes so that biofilm cells are clearly phenotypically distinct from their planktonic counterparts. Each biofilm bacterium lives in a customized microniche in a complex microbial community that has primitive homeostasis, a primitive circulatory system, and metabolic cooperativity, and each of these sessile cells reacts to its special environment so that it differs fundamentally from a planktonic cell of the same species.
The biofilm matrix—An immobilized but dynamic microbial environment
[J].The biofilm matrix is a dynamic environment in which the component microbial cells appear to reach homeostasis and are optimally organized to make use of all available nutrients. The major matrix components are microbial cells, polysaccharides and water, together with excreted cellular products. The matrix therefore shows great microheterogeneity, within which numerous microenvironments can exist. Although exopolysaccharides provide the matrix framework, a wide range of enzyme activities can be found within the biofilm, some of which will greatly affect structural integrity and stability.
Bacterial biofilms: From the natural environment to infectious diseases
[J].Biofilms--matrix-enclosed microbial accretions that adhere to biological or non-biological surfaces--represent a significant and incompletely understood mode of growth for bacteria. Biofilm formation appears early in the fossil record (approximately 3.25 billion years ago) and is common throughout a diverse range of organisms in both the Archaea and Bacteria lineages, including the 'living fossils' in the most deeply dividing branches of the phylogenetic tree. It is evident that biofilm formation is an ancient and integral component of the prokaryotic life cycle, and is a key factor for survival in diverse environments. Recent advances show that biofilms are structurally complex, dynamic systems with attributes of both primordial multicellular organisms and multifaceted ecosystems. Biofilm formation represents a protected mode of growth that allows cells to survive in hostile environments and also disperse to colonize new niches. The implications of these survival and propagative mechanisms in the context of both the natural environment and infectious diseases are discussed in this review.
Concise review of mechanisms of bacterial adhesion to biomaterial surfaces
[J].
Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications
[J].This review covers the most recent developments of inorganic and organic-inorganic composite coatings for orthopedic implants, providing the interface with living tissue and with potential for drug delivery to combat infections. Conventional systemic delivery of drugs is an inefficient procedure that may cause toxicity and may require a patient's hospitalization for monitoring. Local delivery of antibiotics and other bioactive molecules maximizes their effect where they are required, reduces potential systemic toxicity and increases timeliness and cost efficiency. In addition, local delivery has broad applications in combating infection-related diseases. Polymeric coatings may present some disadvantages. These disadvantages include limited chemical stability, local inflammatory reactions, uncontrolled drug-release kinetics, late thrombosis and restenosis. As a result, embedding of bioactive compounds and biomolecules within inorganic coatings (bioceramics, bioactive glasses) is attracting significant attention. Recently nanoceramics have attracted interest because surface nanostructuring allows for improved cellular adhesion, enhances osteoblast proliferation and differentiation, and increases biomineralization. Organic-inorganic composite coatings, which combine biopolymers and bioactive ceramics that mimick bone structure to induce biomineralization, with the addition of biomolecules, represent alternative systems and ideal materials for "smart" implants. In this review, emphasis is placed on materials and processing techniques developed to advance the therapeutic use of biomolecules-eluting coatings, based on nanostructured ceramics. One part of this report is dedicated to inorganic and composite coatings with antibacterial functionality.Inorganic and composite nanotechnology-based coating methods have recently been developed for orthopedic applications, with the main goal to provide bactericide and other enhanced properties, which may result in reduced need for pharmaceutical interventions and overall more cost effective orthopedic procedures. This review discusses key aspects of the above developments.Copyright © 2011 Elsevier Inc. All rights reserved.
Research progress in antibacterial stainless steel
[J].
不锈钢抗菌技术研究进展
[J].
Research progress of antibacterial stainless steel
[J].
抗菌不锈钢的研究进展
[J].
Competition between abiogenic and biogenic metal cations in biological systems: Mechanisms of gallium's anticancer and antibacterial effect
[J].
Gallium complexes as anticancer drugs
[A].
Theoretical study of gallium nitride nanocage as a carrier for 5-fluorouracil anticancer drug
[J].In this paper, the possible interactions between 5-fluorouracil (5FU) as an anticancer drug and gallium nitride (GaN) nanocage (NC) in aqueous solution have been investigated using DFT/CPCM/B3LYP-D/6-31G(d,p) level of theory. Eleven different orientations were used to mimic the 5FU adsorbed on GaN (5FU@GaNNC). To investigate the interaction mechanism between the two components, the adsorption energies and thermodynamic parameters, the electronic properties such as the energies and orbitals distribution of the highest occupied molecular orbital (HOMO), the lowest unoccupied molecular orbital (LUMO), the HOMO-LUMO energy gaps (E), the density of states (DOS), partial DOS (PDOS), and the molecular electrostatic potential (MEP) have been calculated and compared. The natural bond orbitals (NBOs) and the quantum theory of atoms in molecules (QTAIM) calculations have been applied for understanding chemical interactions and chemical bonding. Additionally, some quantum molecular descriptors were calculated for the understanding of molecular reactivity. Main results revealed that (1) the key factor that leads to stabilization of the formed complex/s is the relocation of one of the H atoms that originally belonging to one of the N atoms in 5FU to one of the nearest Ga atoms in GaNNC and (2) the adsorption energies for the eleven adsorbed systems are relatively larger compared with reported similar systems indicating from a theoretical point of view, a probable chemisorption type of adsorption and the privilege of GaNNC as a carrier for 5FU drug. Graphical abstract Simulation of the most stable adsorbed system of 5-fluorouracil anticancer drug on Gallium nitride nanocage.
A gallium(III) complex that engages protein disulfide isomerase A3 (PDIA3) as an anticancer target
[J].
Structurally characterized gallium-chrysin complexes with anticancer potential
[J].Chemotherapeutic metal-based compounds are effective anticancer agents; however, their cytotoxic profile and significant side effects limit their wide application. Natural products, especially flavonoids, are a prominent alternative source of anticancer agents that can be used as ligands for the generation of new bioactive complexes with metal ions of known biochemical and pharmacological activities. Herein, we present the synthesis and detailed structural and physicochemical characterizations of three novel complex assemblies of Ga(iii) with the flavonoid chrysin and the ancillary aromatic chelators 1,10-phenanthroline, 2,2'-bipyridine and imidazole. The complexes constitute the only crystallographically characterized structures having a metal core from the boron group elements and a flavonoid as the ligand. The in vitro biological evaluation of the three complexes in a series of cancer cell lines of different origin established their cytotoxicity and ROS generating potential. In particular, the Ga(iii)-chrysin-imidazole complex displayed the highest anticancer efficacy against all cancer cell lines with IC50 values in the low micromolar range (<1.18 μM), a result worth further investigation.
Gallium in bacteria, metabolic and medical implications
[A].
Promises and failures of gallium as an antibacterial agent
[J].Gallium has a long history as a diagnostic and chemotherapeutic agent. The pharmacological properties of Ga(III) rely on chemical mimicry; when Ga(III) is exogenously supplied to living cells it can replace Fe(III) within target molecules, thereby perturbing bacterial metabolism. Ga(III)-induced metabolic distresses are dramatic in fast-growing cells, like bacterial cells. Interest in the antibacterial properties of Ga(III) has been raised by the compelling need for novel drugs to combat multidrug-resistant bacteria and by the shortage of new antibiotic candidates in the pharmaceutical pipeline. Ga(III) activity has been demonstrated, both in vitro and in animal models of infections, on several bacterial pathogens, also including intracellular and biofilm-forming bacteria. Ga(III) activity is affected by iron availability and the metabolic state of the cell, being maximal in iron-poor media and in respiring cells. Synergism between Ga(III) and antibiotics holds promise as last resort therapy for infections sustained by pandrug-resistant bacteria.
Gallium as a potential candidate for treatment of osteoporosis
[J].Gallium (Ga) is a semi-metallic element that displays antitumor, antiresorptive, anti-inflammatory and immunosuppressive properties. Among all these properties, antitumor properties were the most extensively applied and have shown efficacy in treatment of Paget's disease, myeloma and hypercalcemia in cases of malignancy. By contrast, no clinical trials have been conducted in prevention and/or treatment of osteoporosis. In this article I focus on Ga effects on bone tissue and cells, as well as on molecular mechanisms governing Ga internalization into cells. Eventually, the potential of Ga as an antiosteoporotic agent is discussed.Copyright © 2012 Elsevier Ltd. All rights reserved.
The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity
[J].
Gallium disrupts iron metabolism of mycobacteria residing within human macrophages
[J].Mycobacterium tuberculosis and M. avium complex (MAC) enter and multiply within monocytes and macrophages in phagosomes. In vitro growth studies using standard culture media indicate that siderophore-mediated iron (Fe) acquisition plays a critical role in the growth and metabolism of both M. tuberculosis and MAC. However, the applicability of such studies to conditions within the macrophage phagosome is unclear, due in part to the absence of experimental means to inhibit such a process. Based on the ability of gallium (Ga(3+)) to concentrate within mononuclear phagocytes and on evidence that Ga disrupts cellular Fe-dependent metabolic pathways by substituting for Fe(3+) and failing to undergo redox cycling, we hypothesized that Ga could disrupt Fe acquisition and Fe-dependent metabolic pathways of mycobacteria. We find that Ga(NO(3))(3) and Ga-transferrin produce an Fe-reversible concentration-dependent growth inhibition of M. tuberculosis strains and MAC grown extracellularly and within human macrophages. Ga is bactericidal for M. tuberculosis growing extracellularly and within macrophages. Finally, we provide evidence that exogenously added Fe is acquired by intraphagosomal M. tuberculosis and that Ga inhibits this Fe acquisition. Thus, Ga(NO(3))(3) disruption of mycobacterial Fe metabolism may serve as an experimental means to study the mechanism of Fe acquisition by intracellular mycobacteria and the role of Fe in intracellular survival. Furthermore, given the inability of biological systems to discriminate between Ga and Fe, this approach could have broad applicability to the study of Fe metabolism of other intracellular pathogens.
Antimicrobial activity of gallium against virulent Rhodococcus equiin vitro and in vivo
[J].Rhodococcus equi, a facultative intracellular bacterium, causes severe pneumonia in foals. Evidence suggests that most foals become infected very early in life, when they have immature or ineffective innate immune responses. This study evaluated the antimicrobial activity of gallium against R. equi, as a potential chemoprophylactic and therapeutic agent. Rhodococcus equi was grown in media with various concentrations of gallium nitrate (GN), with and without excess iron. GN significantly inhibited growth and killed R. equi, and these effects were abolished with excess iron. Antimicrobial effects of Ga appear to be related to its interference with iron metabolism. Mice were treated orally with gallium maltolate (GaM), 10 or 50 mg/kg BW, or distilled H2O prior to and after experimental infection with R. equi. Six days post-infection, organs were harvested and R. equi concentrations assessed, and serum gallium concentrations determined. GaM was absorbed in a dose-dependent manner, and R. equi tissue burdens were greater in control mice than in all GaM-treated mice. GaM may aid in the control of disease by preventing development of overwhelming R. equi tissue burdens prior to the establishment of requisite innate and adaptive immune responses.
In vitro antimicrobial activity of gallium maltolate against virulent Rhodococcus equi
[J].
In vitro activity of gallium maltolate against staphylococci in logarithmic, stationary, and biofilm growth phases: Comparison of conventional and calorimetric susceptibility testing methods
[J].Ga(3+) is a semimetal element that competes for the iron-binding sites of transporters and enzymes. We investigated the activity of gallium maltolate (GaM), an organic gallium salt with high solubility, against laboratory and clinical strains of methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA), methicillin-susceptible Staphylococcus epidermidis (MSSE), and methicillin-resistant S. epidermidis (MRSE) in logarithmic or stationary phase and in biofilms. The MICs of GaM were higher for S. aureus (375 to 2000 microg/ml) than S. epidermidis (94 to 200 microg/ml). Minimal biofilm inhibitory concentrations were 3,000 to >or=6,000 microg/ml (S. aureus) and 94 to 3,000 microg/ml (S. epidermidis). In time-kill studies, GaM exhibited a slow and dose-dependent killing, with maximal action at 24 h against S. aureus of 1.9 log(10) CFU/ml (MSSA) and 3.3 log(10) CFU/ml (MRSA) at 3x MIC and 2.9 log(10) CFU/ml (MSSE) and 4.0 log(10) CFU/ml (MRSE) against S. epidermidis at 10x MIC. In calorimetric studies, growth-related heat production was inhibited by GaM at subinhibitory concentrations; and the minimal heat inhibition concentrations were 188 to 4,500 microg/ml (MSSA), 94 to 1,500 microg/ml (MRSA), and 94 to 375 microg/ml (MSSE and MRSE), which correlated well with the MICs. Thus, calorimetry was a fast, accurate, and simple method useful for investigation of antimicrobial activity at subinhibitory concentrations. In conclusion, GaM exhibited activity against staphylococci in different growth phases, including in stationary phase and biofilms, but high concentrations were required. These data support the potential topical use of GaM, including its use for the treatment of wound infections, MRSA decolonization, and coating of implants.
Gallium and its competing roles with iron in biological systems
[J].Gallium, a group IIIa metal, shares chemical properties with iron. Studies have shown that gallium-based compounds have potential therapeutic activity against certain cancers and infectious microorganisms. By functioning as an iron mimetic, gallium perturbs iron-dependent proliferation processes in tumor cells. Gallium's action on iron homeostasis leads to disruption of ribonucleotide reductase, mitochondrial function, and the regulation of transferrin receptor and ferritin. In addition, gallium nitrate stimulates an increase in mitochondrial reactive oxygen species in cells which triggers downstream upregulation of metallothionein and hemoxygenase-1. Gallium's anti-infective activity against bacteria and fungi results from disruption of microbial iron utilization through mechanisms which include gallium binding to siderophores and downregulation of bacterial iron uptake. Gallium compounds lack cross-resistance to conventional chemotherapeutic drugs and antibiotics thus making them attractive agents for drug development. This review will focus on the mechanisms of action of gallium with emphasis on its interaction with iron and iron proteins.Copyright © 2016 Elsevier B.V. All rights reserved.
In vitro clearance effects of gallium nitrate on biofilms of clinically isolated Staphylococcus aureus
[J].
硝酸镓对临床分离金黄色葡萄球菌生物膜的体外清除作用
[J].
The biofilm formation onto implants and prosthetic materials may be contrasted using gallium (3+)
[J].
Gallium maltolate has in vitro antiviral activity against SARS-CoV-2 and is a potential treatment for COVID-19
[J].
Evaluation on the cytotoxicity of gallium alloy by MTT-assay
[J].To test the cytotoxicity of Gallium alloy, a new mercury-free dental restorative material.L-929 mouse fibroblasts were used to detect the cell relative proliferation rate of Gallium alloy and high-copper amalgam by MTT-assay.The results indecated that the relative growth rate induced by Gallium alloy was high (93.0% +/- 4.9% 2ds, 102.0% +/- 3.5% 4ds, 107.2% +/- 4.2% 7ds), and the cytotoxicity of Gallium alloy was 0 grade according to the Test Standard of Shanghai Medical Biomaterial, meaning Gallium alloy had no significant cytotoxicity and the relative growth rate of Gallium alloy was higher than that of high-copper amalgam. The high-copper amalgam showed medium cytotoxicity.Gallium alloy has no significant cytotoxicity.
MTT法评价镓合金的细胞毒性
[J].
Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections
[J].
Superior antibacterial activity of gallium based liquid metals due to Ga3+ induced intracellular ROS generation
[J].
Metallurgical gallium additions to titanium alloys demonstrate a strong time-increasing antibacterial activity without any cellular toxicity
[J].
Iron/heme metabolism-targeted gallium(III) nanoparticles are active against extracellular and intracellular Pseudomonas aeruginosa and Acinetobacter baumannii
[J].
Quantitative proteomic reveals gallium maltolate induces an iron-limited stress response and reduced quorum-sensing in Pseudomonas aeruginosa
[J].
Magnetostrictive alloys: Promising materials for biomedical applications
[J].Magnetostrictive alloys have attracted increasing attention in biomedical applications because of the ability to generate reversible deformation in the presence of external magnetic fields. This review focuses on the advances in magnetostrictive alloys and their biomedical applications. The theories of magnetostriction are systematically summarized. The different types of magnetostrictive alloys and their preparation methods are also reviewed in detail. The magnetostrictive strains and phase compositions of typical magnetostrictive alloys, including iron based, rare-earth based and ferrite materials, are presented. Besides, a variety of approaches to preparing rods, blocks and films of magnetostriction materials, as well as the corresponding methods and setups for magnetostriction measurement, are summarized and discussed. Moreover, the interactions between magnetostrictive alloys and cells are analyzed and emphasis is placed on the transduction and transformation process of mechanochemical signals induced by magnetostriction. The latest applications of magnetostrictive alloys in remote microactuators, magnetic field sensors, wireless implantable devices and biodegradable implants are also reviewed. Furthermore, future research directions of magnetostrictive alloys are prospected with focus on their potential applications in remote cell actuation and bone repair.© 2021 The Authors.
A Zn-doped CuO nanocomposite shows enhanced antibiofilm and antibacterial activities against Streptococcus mutans compared to nanosized CuO
[J].
Predicting the variation of stacking fault energy for binary Cu alloys by first-principles calculations
[J].The variation of stacking fault energy (SFE) in a number of binary Cu alloys is predicted through considering the Suzuki segregation by the full potential linearly augmented plane wave (FPLAPW) method. The calculated results show that some solute atoms (Mg, Al, Si, Zn, Ga, Ge, Cd, Sn, and Pb), which prefer to form the Suzuki segregation, may decrease the value of SFE; while the others (Ti, Mn, Fe, Ni, Zr, Ag, and Au), which do not cause the Suzuki segregation may not decrease the SFE. Furthermore, it is interesting to find that the former alloying elements are located on the right of Cu group while the latter on the left of Cu group in the periodic table of elements. The intrinsic reasons for the new findings can be traced down to the valences electronic structure of solute and Cu atoms, i.e., the similarity of valence electronic structure between solute and Cu atoms increases the value of SFE, while the difference decreases the value of SFE.
Gallium(III) ion hydrolysis under physiological conditions
[J].
Solubilities of metal hydroxides
[J].
The some physicochemical studies on Gallium(III) salt solutions
[J].
Gallium-protoporphyrin IX inhibits Pseudomonas aeruginosa growth by targeting cytochromes
[J].
New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine-iron uptake pathway
[J].
gallium and their impact on oxidative stress
[J].The role metals play in living organisms is well established and subject to extensive research. Some of them participate in electron-exchange reactions. Such reactions cause generation of free radicals that can adversely impact biological systems, as a result of oxidative stress. The impact of 'non-biological' metals on oxidative stress is also a worthy pursuit due to the crucial role they play in modern civilization. Lanthanides (Ln) are widely used in modern technology. As a result, human exposure to them is increasing. They have a number of established medical applications and are being extensively researched for their potential antiviral, anticancer and anti-inflammatory properties. The present review focuses on lanthanum (La) and its impact on oxidative stress. Another metal, widely used in modern high-tech is gallium (Ga). In some respects, it shows certain similarities to La, therefore it is a subject of the present review as well. Both metals exhibit ionic mimicry which allows them to specifically target malignant cells, initiating apoptosis that makes their simple salts and coordination complexes promising candidates for future anticancer agents.Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects
[J].Titanium embedded with silver nanoparticles (Ag NPs) using a single step silver plasma immersion ion implantation (Ag-PIII) demonstrate micro-galvanic effects that give rise to both controlled antibacterial activity and excellent compatibility with osteoblasts. Scanning electron microscopy (SEM) shows that nanoparticles with average sizes of about 5 nm and 8 nm are formed homogeneously on the titanium surface after undergoing Ag-PIII for 0.5 h and 1 h, respectively. Transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS) indicate that those nanoparticles are metallic silver produced on and underneath the titanium surface via a local nucleation process from the solid solution of α-Ti(Ag). The Ag-PIII samples inhibit the growth of both Staphylococcus aureus and Escherichia coli while enhancing proliferation of the osteoblast-like cell line MG63. Electrochemical polarization and Zeta potential measurements demonstrate that the low surface toxicity and good cytocompatibility are related to the micro-galvanic effect between the Ag NPs and titanium matrix. Our results show that the physico-chemical properties of the Ag NPs are important in the control of the cytotoxicity and this study opens a new window for the design of nanostructured surfaces on which the biological actions of the Ag NPs can be accurately tailored.Copyright © 2010 Elsevier Ltd. All rights reserved.
Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species
[J].Although titanium embedded with silver nanoparticles (Ag-NPs@Ti) are suitable for biomedical implants because of the good cytocompatibility and antibacterial characteristics, the exact antibacterial mechanism is not well understood. In the present work, the antibacterial mechanisms of Ag-NPs@Ti prepared by plasma immersion ion implantation (PIII) are explored in details. The antibacterial effects of the Ag-NPs depend on the conductivity of the substrate revealing the importance of electron transfer in the antibacterial process. In addition, electron transfer between the Ag-NPs and titanium substrate produces bursts of reactive oxygen species (ROS) in both the bacteria cells and culture medium. ROS leads to bacteria death by inducing intracellular oxidation, membrane potential variation, and cellular contents release and the antibacterial ability of Ag-NPs@Ti is inhibited appreciably after adding ROS scavengers. Even though ROS signals are detected from osteoblasts cultured on Ag-NPs@Ti, the cell compatibility is not impaired. This electron-transfer-based antibacterial process which produces ROS provides insights into the design of biomaterials with both antibacterial properties and cytocompatibility.Copyright © 2017 Elsevier Ltd. All rights reserved.