钛合金是一种重要的轻质结构材料,具有密度低、比强度高、耐热性好等优点[1,2],尤其钛合金表面存在致密的钝化膜,使其耐蚀性能十分优异[3,4]。但在实际使用过程中,不可避免地存在一些对钛合金表面钝化膜有破坏性的腐蚀介质,使钛合金部件存在腐蚀风险。Cl-是最常见的对钝化膜破坏性强的离子,但对钛合金而言,当介质中存在Cl-时,钛的腐蚀速率没有明显变化[5],而F-对钛合金钝化膜威胁最大,一旦介质中存在F-,钛合金的耐腐蚀性能会明显降低。在钛合金常见的应用环境中,都含有一定量的F-,例如饮用水中F-的浓度为0.5~0.8 mg/L,而在废水中F-的浓度可达1.5 mg/L以上[6]。此外,医用植入体、管道和海洋船舰[7,8]等一些特殊应用区域,F-浓度可以达到相当高的程度。因此,研究F-对钛合金的腐蚀作用机制非常重要。
在不同pH值环境中,F-对钛合金钝化膜的破坏性存在较大差异,尤其在酸性含F-溶液中钝化膜易发生溶解反应(TiO2 + 6
可见,目前对钛合金在多种腐蚀介质中的腐蚀行为缺乏系统研究。为了更好地明确钛合金在复杂腐蚀环境中的失效过程,本工作探讨了TC4钛合金在含不同离子的3.5%NaCl (质量分数)溶液中的电化学腐蚀行为。
1 实验方法
实验所用材料为TC4钛合金热轧板,主要含6%Al (质量分数,下同)和4%V,余量为Ti。用于金相观察的样品,经水砂纸打磨至2000号,抛光后使用Kroll's试剂(2%HF + 5%HNO3 + 93%H2O,体积分数)常温腐刻1~2 s。采用DSX500光学显微镜(OM)观察样品的显微组织。
电化学测试样品为10 mm×10 mm×10 mm的块状材料,用铜导线与样品连接,保留1 cm2的工作面积,其余部分用树脂封装。样品经过水砂纸逐级打磨至2000号,依次用去离子水和酒精冲洗,冷风吹干,放在干燥器中待用。电化学测试采用三电极体系,测试样品为工作电极,Pt片为辅助电极,饱和甘汞电极(SCE)作为参比电极。采用PARSTAT 4000电化学工作站进行测试。腐蚀介质为3.5%NaCl中加入不同浓度的HCl、NaF及FeCl3,以此调控溶液中的H+、F-和Fe3+浓度(分别表示为cH、cF和cFe)。动电位极化曲线测试的初始延迟时间为1 h,扫描速率为1 mV/s,扫描范围为-0.5~2 V (vs OCP (开路电位)),所获得的极化曲线采用CView软件中的Tafel模式进行拟合。电化学阻抗谱(EIS)的测量参数为:激励信号为幅值10 mV的正弦波,频率为105~10-2 Hz,初始延迟时间为1 h。所有电化学实验均重复3次以上。
2 实验结果
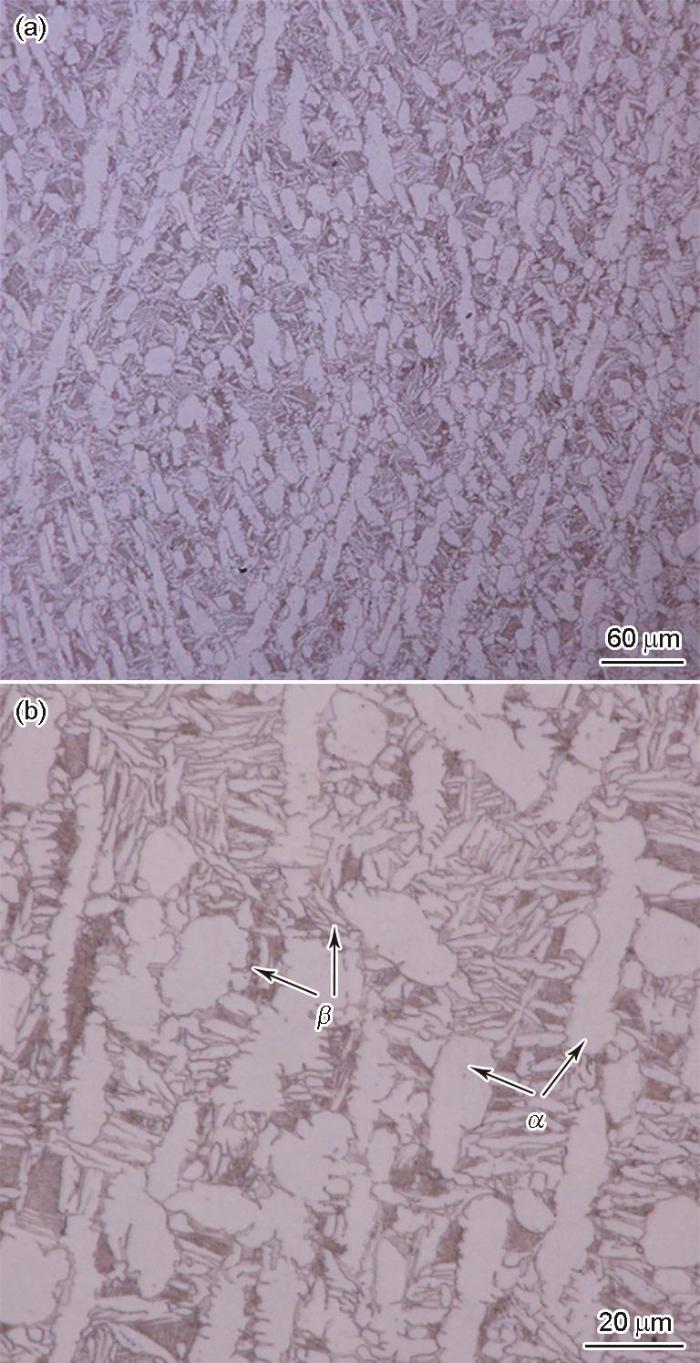
2.1 TC4钛合金的微观结构
图1
图1
TC4钛合金显微组织的OM像
Fig.1
OM images of TC4 alloy with low (a) and high (b) magnifications
2.2 H+ 和F-对TC4钛合金电化学行为的影响
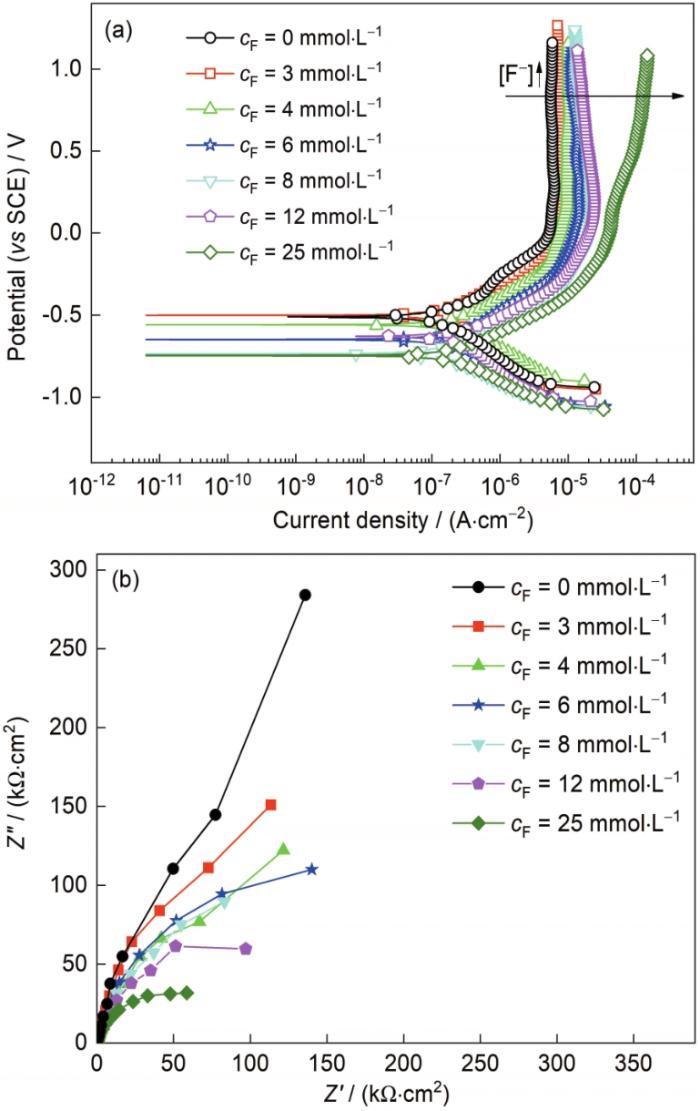
TC4钛合金在含有不同浓度H+的3.5%NaCl中的极化曲线和EIS结果如图2所示。极化曲线拟合结果见表1。从图2a及表1拟合结果可以看出,在不含H+的3.5%NaCl溶液中,阳极保持钝化趋势,在添加H+后仍然表现为自钝化特征;随着H+浓度增加,钝化电流密度(ipp)略有增加,表明H+的存在增大了钝化膜阳极溶解的速率,但增大作用非常微弱。极化曲线的阴极分支发生明显变化,在添加H+后,阴极还原反应速率增加约1个数量级。从含100 mmol/L H+的阴极反应可看出,当阴极极化到较负电位后,有明显氧扩散控制的氧还原反应特征,这表明阴极极化初期电位较正时,主要发生的是氧还原反应,随着电位负移,氧还原反应加快,氧扩散成为控制步骤,在阴极电位达到析氢过电位时,开始发生析氢反应。阴极反应大幅增加的原因包括2个方面,一方面,H+的加入使溶液pH值降低,钛合金表面发生阴极还原反应的动力增大;另一方面,H+对钝化膜有轻微溶解作用,导致钝化膜对阴极反应电子传输的阻挡作用减弱,最终使阴极还原反应速率大幅增加。TC4钛合金在含不同浓度H+的3.5%NaCl中的EIS如图2b所示。可以看出,Nyquist曲线均表现出容抗特征,且容抗弧尺寸并不相同。在未添加H+的3.5%NaCl中的容抗弧尺寸最大,添加H+后容抗弧尺寸减小,这表明钝化膜的阻挡能力在含H+的溶液中略有下降,但不同浓度H+的容抗弧尺寸差异不大。
图2
图2
TC4钛合金在含不同浓度H+的3.5%NaCl中的极化曲线和EIS结果
Fig.2
Potentiodynamic polarazation curves (a) and EIS (b) of TC4 alloy in 3.5%NaCl solutions with different H+ concentrations (cH)
表1 TC4钛合金在含不同浓度H+的3.5%NaCl中极化曲线的拟合结果
Table 1
| cH | Ecorr | icorr | bc | ipp |
|---|---|---|---|---|
| mmol·L-1 | mV | μA·cm-2 | mV·decade-1 | μA·cm-2 |
| 0 | -502 | 0.06 | -223 | 4.48 |
| 25 | -469 | 1.62 | -143 | 4.50 |
| 50 | -469 | 3.14 | -159 | 4.51 |
| 100 | -494 | 4.39 | -204 | 4.51 |
TC4钛合金在含有不同浓度F-的3.5%NaCl中的极化曲线和EIS结果如图3所示,极化曲线拟合结果如表2所示。从图3a及表2拟合结果可见,在含微量F-的中性NaCl中,阳极区钛合金都表现为自钝化行为,但随着F-浓度增加,阳极的ipp随之增大,增幅比在含H+溶液中更明显,尤其在含25 mmol/L F-时,ipp增加一个数量级以上,表现出了从钝化态转变为活化态的趋势,这表明钝化膜的溶解速率随着F-浓度的增加而逐渐加快,这与文献[19]结果一致。但添加F-后对极化曲线的阴极影响比较小,都是发生氧还原反应,且反应速率差异不大。如图3b所示,在不同F-浓度的3.5%NaCl溶液中,TC4钛合金的Nyquist曲线形状都是相似的,呈现明显的容抗特征,随着F-浓度增加,容抗弧尺寸逐渐减小,表明耐蚀性能下降。前期研究[17]结果表明,F-可以参与到钝化膜的溶解反应过程中,随着F-浓度的增加,钝化膜的溶解反应将加快,但钝化膜未完全遭到破坏时,仍能保持钝化特征。
图3
图3
TC4钛合金在含不同浓度F-的3.5%NaCl中的极化曲线和EIS结果
Fig.3
Potentiodynamic polarazation curves (a) and EIS (b) of TC4 alloy in 3.5%NaCl solutions with different F- concentrations (cF)
表2 TC4钛合金在含不同浓度F-的3.5%NaCl中极化曲线的拟合结果
Table 2
| cF | Ecorr | icorr | bc | ipp |
|---|---|---|---|---|
| mmol·L-1 | mV | μA·cm-2 | mV·decade-1 | μA·cm-2 |
| 0 | -502 | 0.06 | -223 | 4.48 |
| 3 | -500 | 0.09 | -207 | 7.65 |
| 4 | -558 | 0.12 | -166 | 9.72 |
| 6 | -648 | 0.19 | -175 | 14.52 |
| 8 | -734 | 0.10 | -169 | 20.97 |
| 12 | -627 | 0.20 | -268 | 20.97 |
| 25 | -744 | 0.13 | -168 | 63.46 |
2.3 H+ 和F-对TC4钛合金电化学行为的协同影响
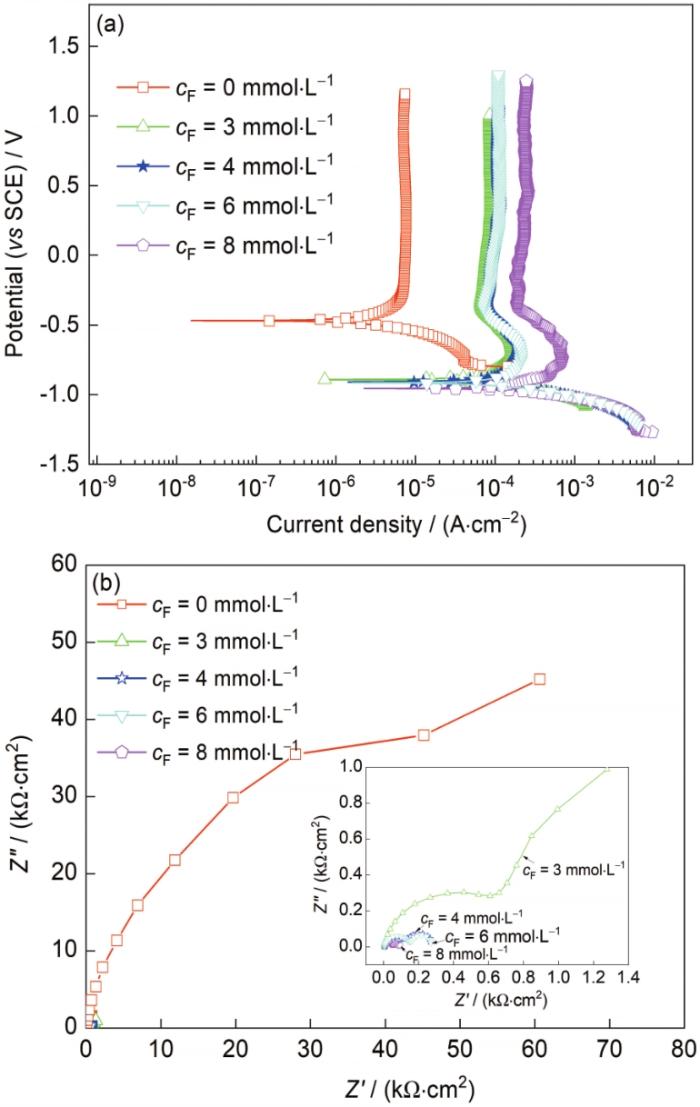
TC4钛合金在含不同浓度F-的酸性3.5%NaCl + 50 mmol/L H+溶液中的极化曲线和EIS结果如图4所示,极化曲线拟合结果见表3。从图4a及表3拟合结果可以看出,当cF = 0 mmol/L时,TC4钛合金表现为自钝化行为;当cF ≥ 3 mmol/L后,TC4钛合金表现出活化-钝化行为,这表明钝化膜处于不稳定状态。同时发现,随着F-浓度的增大,ipp逐渐增大,这表明钝化膜的阳极溶解速率增大,膜层的稳定性下降。此外,阴极分支也发生变化,当cF = 0 mmol/L时,阴极反应为氧还原反应和析氢反应共同控制,当cF ≥ 3 mmol/L后,阴极反应将由析氢反应控制,且析氢反应并不随着F-浓度的改变而发生变化,这与前文献[20]结果一致,F-的存在不影响钛合金的阴极反应过程。TC4钛合金在含不同浓度F-的酸性3.5%NaCl + 50 mmol/L H+中的Nyqusit图如图4b所示。当cF = 0 mmol/L时,Nyqusit图表现为一个单一的容抗弧特征,当cF ≥ 3 mmol/L后,Nyqusit图上出现了2个明显的容抗弧,且随着F-浓度增加,容抗弧半径逐渐减小,这说明钝化膜的致密性下降,耐蚀能力降低。
图4
图4
TC4钛合金在含不同浓度F-的酸性3.5%NaCl + 50 mmol/L H+中的极化曲线和EIS结果
Fig.4
Potentiodynamic polarazation curves (a) and EIS (b) of TC4 alloy in 3.5%NaCl + 50 mmol/L H+ sol-utions with different cF (Inset in Fig.4b shows the EIS of TC4 alloy in 3.5%NaCl + 50 mmol/L H+ solutions with cF of 3, 4, 6, and 8 mmol·L-1)
表3 TC4钛合金在含不同浓度F-的酸性3.5%NaCl + 50 mmol/L H+中极化曲线的拟合结果
Table 3
| cF | Ecorr | icorr | bc | ipp |
|---|---|---|---|---|
| mmol·L-1 | mV | μA·cm-2 | mV·decade-1 | μA·cm-2 |
| 0 | -469 | 3.14 | -159 | 4.48 |
| 3 | -892 | 64.00 | -113 | 89.20 |
| 4 | -908 | 78.27 | -94 | 113.50 |
| 6 | -916 | 130.43 | -104 | 113.50 |
| 8 | -955 | 189.57 | -90 | 239.90 |
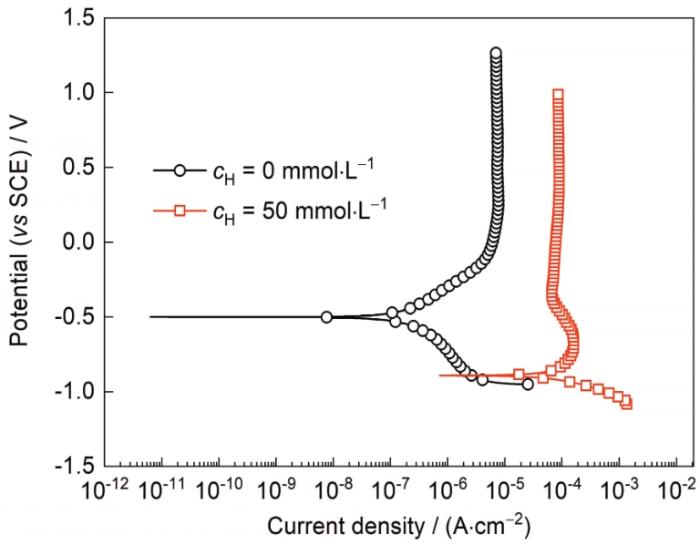
此外,对比了TC4钛合金在F-浓度相同而H+浓度不同的3.5%NaCl溶液中的极化曲线,如图5所示。当H+浓度从cH = 0 mmol/L增加到cH = 50 mmol/L时,极化曲线的阳极分支从完全的钝化行为转变为活化-钝化行为,同时ipp增加一个数量级以上,自腐蚀电位下降约400 mV,阴极的反应速率也大幅增加,这表明在含F-的3.5%NaCl溶液中,增加H + 浓度使阴阳极反应速率都明显加快。
图5
图5
TC4钛合金在含不同浓度H+的3.5%NaCl + 3 mmol/L F-溶液中的极化曲线
Fig.5
Potentiodynamic polarazation curves of TC4 alloy in 3.5%NaCl + 3 mmol/L F- solutions with different cH
对比TC4钛合金在含有不同浓度F-或H+溶液中的极化曲线(图2~5)可以看出,在不同的cF条件下(3、4、6、8 mmol/L F-),增加H+后阳极钝化行为转变为了活化-钝化行为,且在相同cF条件下(3 mmol/L F-),增加H+后阴阳极反应速率都大幅增加。根据前面的分析,F-主要影响TC4钛合金表面的阳极反应,使得表面的钝化性能下降,整体阳极溶解速率逐渐增大(表现为阳极曲线的右移),而对阴极反应没有影响;H+主要影响TC4钛合金表面的阴极反应,使阴极反应从氧还原反应转变成主要为析氢反应,大大加快了阴极反应速率。当F-和H+共同作用时,阳极分支右移的距离远远高于F-单独作用时,因此可以推测:在溶液pH值降低后(添加H+),F-对钝化膜的作用机制发生改变。王政彬[20]根据HF的反应常数推测,pH < 3后,溶液中的F-大部分以HF的形式存在。本实验所用的含50 mmol/L H+的溶液pH值为1.3左右,溶液中同时存在HF和F-。根据文献[21],HF提供的还原环境会抑制钛合金表面氧化膜重新钝化的速率,并且HF对膜层的破坏作用远远高于F-,因此,钝化膜的阳极反应速率将会因HF的存在而大大加快。但本工作中添加的是微量的F-和H+,阳极曲线仍然处于钝化态,样品表面形貌没有明显变化,只是失去了金属光泽,颜色略显发黄。
2.4 Fe3+ 在酸性F-中的缓蚀作用机理
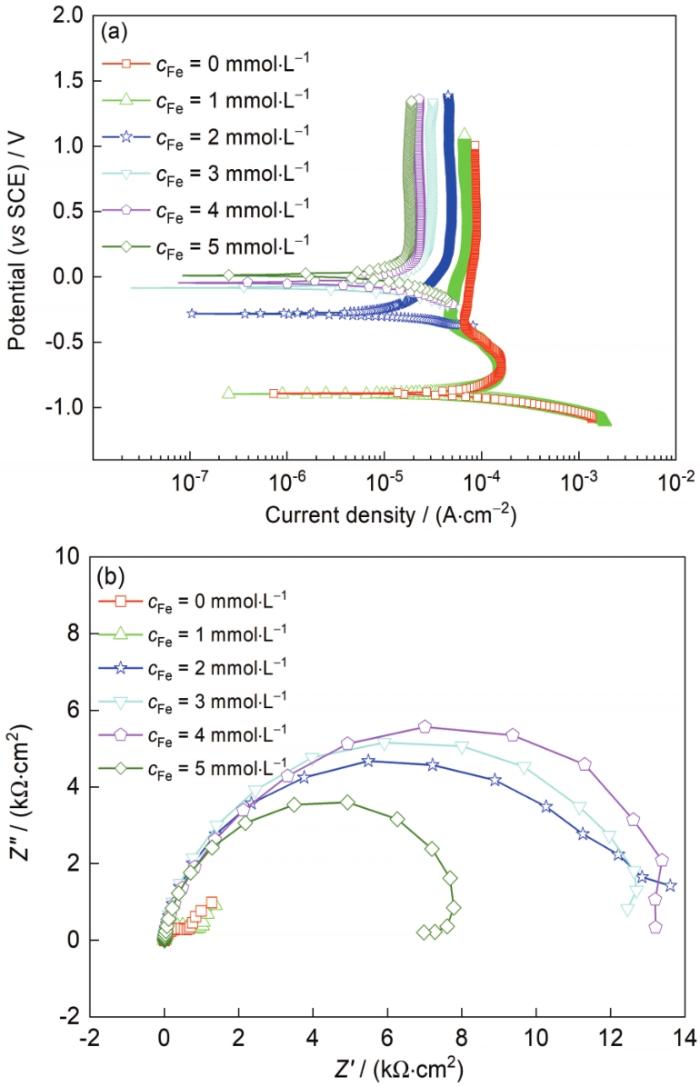
TC4钛合金在含不同浓度Fe3+的酸性含氟溶液3.5%NaCl + 50 mmol/L H+ + 3 mmol/L F-中的极化曲线和EIS结果如图6所示,极化曲线拟合结果见表4。从图6a及表4拟合结果可以看出,在不含Fe3+ (cFe = 0 mmol/L)或cFe < 2 mmol/L的溶液中,阳极曲线呈现活化-钝化行为,表明此时的钝化膜稳定性较差,溶液中的腐蚀性离子可以穿过钝化膜到达基体;而cFe ≥ 2 mmol/L后,阳极曲线表现出自钝化特征,且随着cFe的增加,阳极曲线逐渐向左移动,表明钝化膜溶解速率下降。对于阴极分支,在cFe = 0和cFe = 1 mmol/L时,阴极还原反应为析氢反应控制;而cFe ≥ 2 mmol/L后,阴极反应发生改变。此时,溶液中可能发生的阴极反应有3种:氧还原反应、析氢反应和Fe3+的还原反应。在cFe ≥ 2 mmol/L后,腐蚀电位未达到析氢反应的过电位,因此不考虑析氢反应,只有氧还原反应和Fe3+的还原反应能够发生。当cFe = 2 mmol/L时,由于cFe较低,氧还原反应和Fe3+的还原反应速率相差不大,此时阴极反应由氧还原反应和Fe3+的还原反应共同控制。当cFe > 2 mmol/L时,阴极曲线发生明显的上移,表明此时阴极反应再次发生变化。由于cFe增大,Fe3+还原反应动力增大,此时阴极为Fe3+的还原反应控制。图6b为TC4钛合金在含不同浓度Fe3+的酸性含氟溶液中的Nyquist曲线。可知,在cFe = 0 mmol/L和cFe =1 mmol/L时,Nyquist曲线表现为2个容抗弧,而cFe ≥ 2 mmol/L后,Nyquist曲线表现为单一的容抗弧,且随cFe增大,容抗弧尺寸逐渐增大,这表明在加入Fe3+后膜层的钝化性能增加,且阻挡腐蚀性介质的能力逐渐加强。
图6
图6
TC4钛合金在含不同浓度Fe3+的酸性含氟溶液3.5%NaCl + 50 mmol/L H+ + 3 mmol/L F-中的极化曲线和EIS结果
Fig.6
Potentiodynamic polarazation curves (a) and EIS (b) of TC4 alloy in 3.5%NaCl + 50 mmol/L H+ + 3 mmol/L F- solutions with different Fe3+ concen-trations (cFe)
表4 TC4钛合金在含不同浓度Fe3+的酸性含氟溶液3.5%NaCl + 50 mmol/L H+ + 3 mmol/L F-中极化曲线的拟合结果
Table 4
| cFe | Ecorr | icorr | bc | ipp |
|---|---|---|---|---|
| mmol·L-1 | mV | μA·cm-2 | mV·decade-1 | μA·cm-2 |
| 0 | -892 | 64.00 | -113 | 89.2 |
| 1 | -892 | 64.00 | -113 | 72.4 |
| 2 | -450 | 9.57 | -121 | 43.1 |
| 3 | -84 | 8.68 | -179 | 30.0 |
| 4 | -43 | 7.83 | -179 | 23.6 |
| 5 | 11 | 2.64 | -206 | 18.2 |
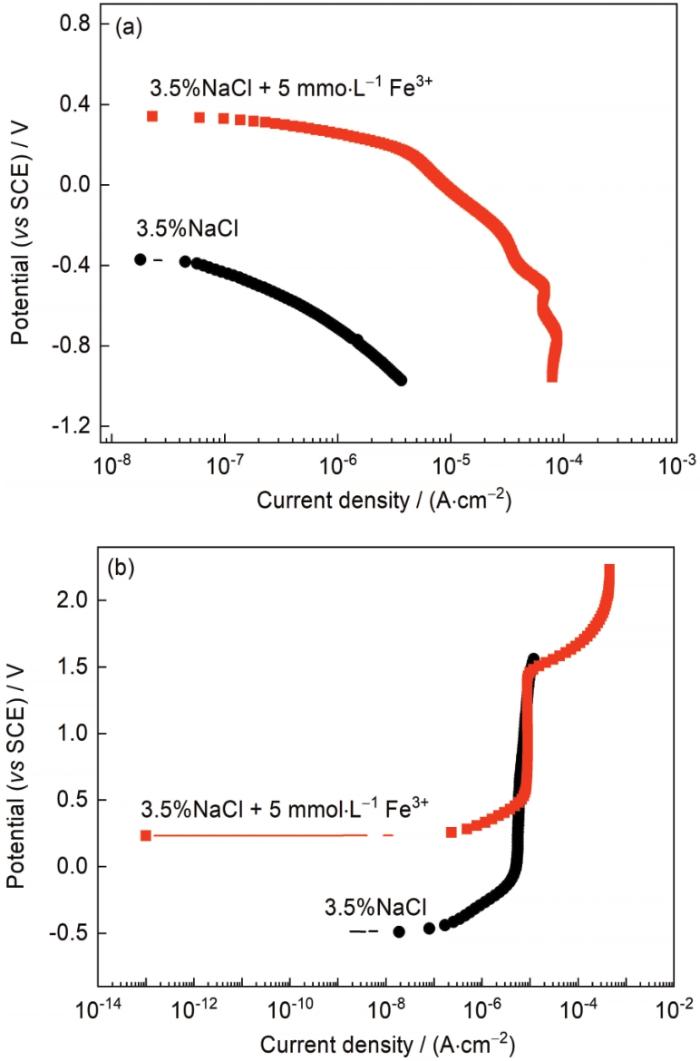
图7
图7
Fe3+对TC4钛合金在3.5%NaCl中阴极和阳极极化曲线的影响
Fig.7
Effects of Fe3+ on the cathodic (a) and anodic (b) polarazation curves of TC4 alloy in 3.5%NaCl solution
3 分析与讨论
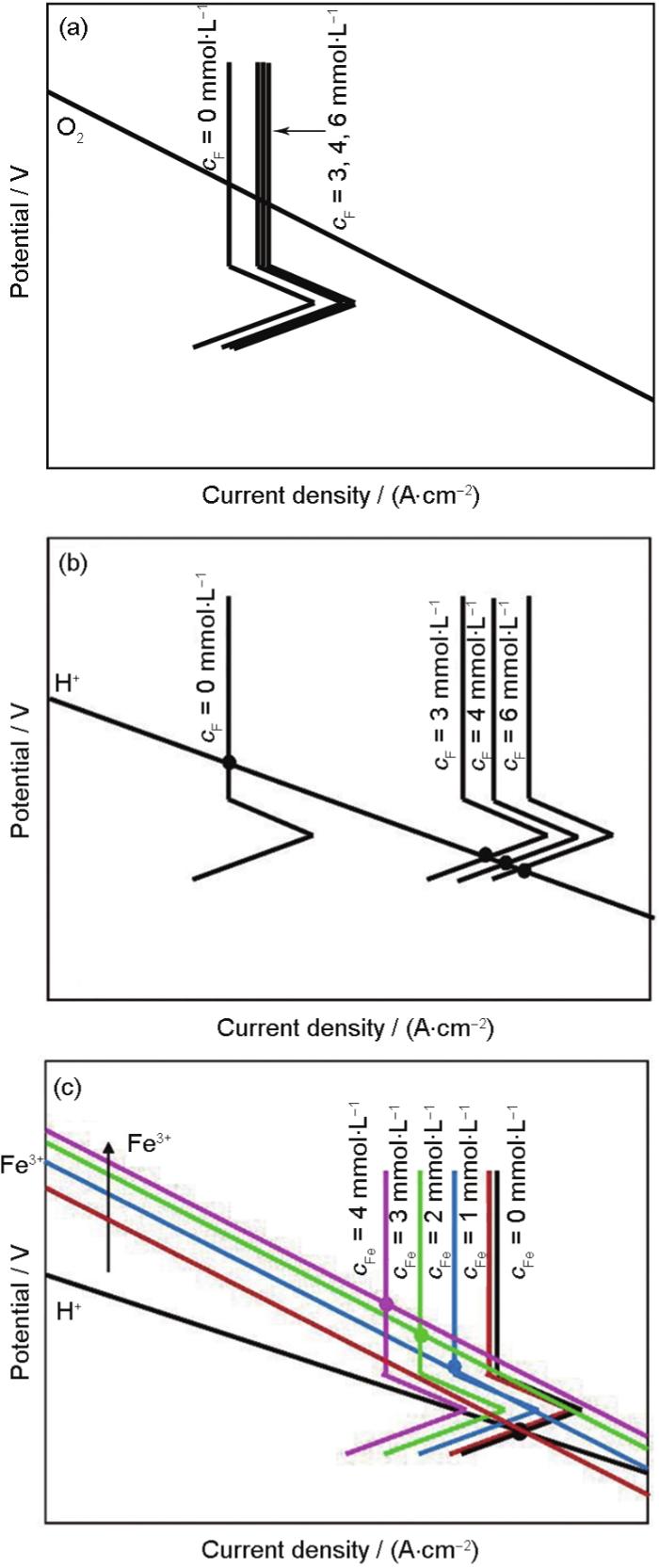
图8
图8
不同离子对钛合金极化曲线影响示意图
Fig.8
Sketch maps of the effect of different ions on the polarazation curves of Ti alloy
(a) F- (b) H+ + F- (c) H+ + F-+ Fe3+
在添加H+的酸性溶液中,参与阴极的反应通常有氧还原和析氢2种:
通过Nernst方程可以计算出2个反应的平衡电位(Ee)与溶液中H+浓度(pH值)的关系:
根据上述公式,氧还原反应的平衡电位始终在析氢反应的平衡电位之上。通常情况下,钛合金阴极发生氧还原反应。然而,当溶液中存在H+后,随着H+浓度增加(pH值减小),Ee2将增加,析氢反应的驱动力增加,析氢反应将不能忽略。
在F-和H+同时存在的介质中,HF会导致钝化膜溶解加快。因此,酸性氟溶液中钝化膜的溶解速率将远大于中性氟溶液。阳极曲线右移程度大大增加,相应的示意图如图8b所示。
缓蚀剂Fe3+对钛合金在酸性F-中的电化学过程影响分为2个方面。其一,介质中能参与阴极还原反应的离子增加,除上述的氧还原和析氢反应外,Fe3+/Fe2+氧化还原对也参与到阴极反应中:
其平衡电位可以表示为:
式中,E0为标准电位,只与温度和压力有关;R为理想气体常数,8.314 J/(K·mol);T为热力学温度,K;F为Faraday常数,96485 C/mol;[Fe3+]和[Fe2+]分别表示Fe3+和Fe2+的浓度,mol/L。Fe3+/Fe2+氧化还原对具有快速反应动力和高的平衡电位[24]。同时,根据Fe(OH)3的溶解积Ksp:
在25℃时,根据手册数据[25]可知,Ksp为3 × 10-39。根据H2O的离子积常数Kw,当[H+] = 100 mmol/L时,OH-的浓度[OH-] = 10-13。计算得出,加入的Fe3+将全部以离子形态存在。也就是说,随着加入Fe3+浓度的增加,[Fe3+]逐渐增大。Liu等[16]通过计算得出:25℃条件下,在[Fe3+]和[Fe2+]皆为1 mmol/L时Ee3的值大约为0.56 V。该平衡电位远远大于Ee2的值([H+] = 100 mmol/L时为-0.059 V)。根据
4 结论
(1) H+的存在对TC4钛合金阳极钝化行为影响较小,主要起到加速阴极反应的作用;F-的存在对阳极钝化行为影响较大,随着F-浓度增加,TC4钛合金表面钝化膜的溶解速率逐渐增加,但F-对阴极还原反应影响较小;当F-和H+共同存在时2者协同作用,大大加速了钝化膜的阳极溶解速率及阴极还原反应速率。
(2) 基于混合电位理论,提出了Fe3+对钛合金在酸性F-中的缓蚀机理。随着Fe3+的浓度增加,Fe3+的阴极还原反应加速,使阴、阳极曲线的交点从活化-钝化区转移到钝化区;同时,溶液中Fe3+通过络合作用降低游离态F-的浓度,减弱F-对钝化膜的破坏作用,最终,2者协同影响大大降低了钝化膜的溶解速率。
参考文献
Effect of hot deformation parameters on the evolution of microstructure and texture of β phase in TC18 titanium alloy
[J].
热变形参数对TC18钛合金β相组织及织构演变规律的影响
[J].利用SEM及EBSD技术,研究热变形参数(变形方式、变形温度、变形量、应变速率、保温时间)对TC18钛合金β相组织及织构演变规律的影响。结果表明,TC18钛合金在热压缩及两相区热拉伸时,β相均以动态回复为主。在热压缩后,主要形成{100}及{111}织构,在热拉伸后,主要形成{110}织构;在单相区压缩时,随着变形温度升高、变形量提高、应变速率降低,{100}织构比例提高、{111}织构比例降低;在两相区压缩时,随着变形温度升高、变形量提高,{100}织构比例提高、{111}织构比例降低;在两相区拉伸时,随着变形量提高,{110}织构比例逐渐提高。
Effect of ZrN modified layer on friction and wear properties of TA18 titanium alloy
[J].
ZrN改性层对TA18钛合金摩擦磨损性能的影响
[J].
Electrochemical performance of passive film pormed on Ti-Al-Nb-Zr alloy in simulated deep sea environments
[J].
Impact of equal channel angular pressing on mechanical behavior and corrosion resistance of hot-rolled Ti-2Fe-0.1B alloy
[J].In the present study, the unique bimodal grain size distribution microstructure with the ultrafine substrate and embedded macro grains was fabricated by a traditional hot-rolling process in a novel low-cost Ti-2Fe-0.1B titanium alloy, which possesses a good combination of strength (around 663 MPa) and ductility (around 30%) without any post heat treatment. Meanwhile, the mechanical behavior and corrosion resistance of hot-rolled Ti-2Fe-0.1B alloy after equal channel angular pressing (ECAP) deformation were studied. Results indicated that the average grain size decreased to 0.24 μm after 4 passes ECAP deformation, which led to the enhancement of tensile strength to around 854 MPa and good ductility to around 15%. In addition, corrosion resistance was also improved after ECAP due to the rapid self-repairing and thicker passivation film. Our study revealed that the novel low-cost titanium alloy after hot-rolling and ECAP could be used instead of Ti-6Al-4V in some industrial applications due to similar mechanical behavior and better corrosion resistance.
Effect of passive film on the galvanic corrosion of titanium alloy Ti60 coupled to copper alloy H62
[J].
Treatment of high fluoride-content wastewater by continuous electrocoagulation-flotation system with bipolar aluminum electrodes
[J].
Research progress on crevice corrosion, plasma nitriding and surface nanocrystallization of titanium alloys
[J].
钛合金缝隙腐蚀、离子渗氮与表面纳米化的研究进展
[J].
Corrosion of titanium alloys in high temperature near anaerobic seawater
[J].
Effect of HF concentration on anodic dissolution of titanium
[J].
The effect of fluoride ions on the corrosion behavior of pure titanium in 0.05 M sulfuric acid
[J].
Influence of fluoride concentration and pH on corrosion behavior of titanium in artificial saliva
[J].
The electrochemistry of titanium corrosion
[D].
Anodic behavior of titanium and commercial alloys in sulfuric acid
[J].The anodic polarization behavior of commercially pure alpha titanium 75A (annealed), beta alloy 13V-llCr-3Al (annealed) and alpha-beta alloy 6Al-6V-2Sn (heat treated to strength levels of 140, 160 and 180 ksi) in hydrogen saturated 5, 12 and 20 percent sulfuric acid solutions was investigated at 20, 35, 50 and 65 ± 1 C, using a potentiostatic technique.
Resistance of titanium to sulfuric and hydrochloric acids inhibited by ferric and cupric ions
[J].
The mechanism of passivating-type inhibitors
[J].
Effect of Fe(III) and Cu(II) on the passivation of Ti-2 in acidic chloride solutions
[J].The passivation of Ti-2 by Fe(III) and Cu(II) species in acidic chloride solutions was investigated by electrochemical techniques. It was found that the critical concentrations of Fe (III) and Cu(II) species required to induce passivation of Ti-2 increased from 1.0 +/- 0 to 6.0 +/- 0 mM and from 0.25 +/- 0 to 1.15 +/- 0.23 mM, respectively, when the temperature was increased from 30 degrees C to 80 degrees C. The mechanism associated with the passivation was the acceleration of the cathodic reactions due to the introduction of oxidants for Ti-2. Cu(II) was more effective than Fe(III) at inducing the passivation of Ti-2 for the conditions investigated here. (C) 2019 The Electrochemical Society.
Destruction of few fluorides to passive film of Ti-6Al-4V alloy under oxygen deficiency crevice conditions
[J].
Corrosion behavior of dual-phase Ti-6Al-4V alloys: A discussion on the impact of element Fe
[J].
The effect of fluoride on electrochemical corrosion of the dental pure titanium before and after adhesion of Streptococcus mutans
[J].To investigate the effect of fluoride on electrochemical corrosion of the dental pure titanium before and after adhesion of Streptococcus mutans.The dental pure titanium specimens were tested by electrochemical measurement system including electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization curve (PD) methods in artificial saliva with 0 g/L and 1.0 g/L sodium fluoride before and after dipped into culture medium with Streptococcus mutans for 24 h. The corrosion parameters, including the polarization resistance (R(ct)), corrosion potential (E(corr)), pitting breakdown potential (E(b)), and the difference between E(corr) and E(b) representing the "pseudo-passivation" (ΔE) obtained from the electrochemical tests were used to evaluate the corrosion resistance of dental pure titanium. The data were statistically analyzed by 2×2 factorial statistical analysis to examine the effect of sodium fluoride and adhesion of Streptococcus mutans using SPSS 12.0 software package.The results showed that the corrosion parameters including R(ct), Ecorr, E(b), and ΔE of pure titanium had significant difference between before and after adhesion of Streptococcus mutans in the same solution(P<0.05), and in artificial saliva with 0 g/L and 1.0 g/L sodium fluoride(P<0.05).The dental pure titanium was prone to corrosion in artificial saliva with sodium fluoride. The corrosion resistance of pure titanium decreased distinctly after immersed in culture medium with Streptococcus mutans.
氟对变形链球菌黏附前后纯钛表面腐蚀性能的影响
[J].
Study of the critical fluoride concentration for the corrosion of pure titanium and its protective measurements in desulfurized wet flue gas environment
[D].
脱硫湿烟气环境下纯钛腐蚀的临界氟离子浓度现象及防护措施研究
[D].
Stability of cp-Ti and Ti-6Al-4V alloy for dental implants as a function of saliva pH—An electrochemical study
[J].
Effects of fluorine on photocatalysis
[J].Tailoring the microstructure of pristine TiO<sub>2</sub> is essential to narrow its band gap and prolong the charge lifetime. In particular, strategies involving fluorine have been used successfully to tune the surface chemistry, electronic structure, and morphology of TiO<sub>2</sub> photocatalysts to improve their photocatalytic activity based on the strong complexation between fluoride ions and TiO<sub>2</sub> and the high electronegativity of fluorine. In this review, we summarize the strategies involving fluorine to establish highly efficient TiO<sub>2</sub> photocatalytic systems or fabricate highly efficient TiO<sub>2</sub> photocatalysts. The main fluorine effects (i.e. the effects of fluorine on photocatalysis) include the following four aspects:(1) Surface effects of fluoride on TiO<sub>2</sub> photocatalysis, (2) effects of fluorine doping on TiO<sub>2</sub> photocatalysis, (3) fluoride-mediated tailoring of the morphology of TiO<sub>2</sub> photocatalysts, and (4) the effects of fluorine on non-TiO<sub>2</sub> photocatalysis. Additionally, the unique applications of these fluorine effects in photocatalysis, including selective degradation of pollutants, selective oxidation of chemicals, water-splitting to produce H<sub>2</sub>, reduction of CO<sub>2</sub> to produce solar fuels, and improvement of the thermostability of TiO<sub>2</sub> photocatalysts, are reviewed.
Effects of fluoride concentration and elastic tensile strain on the corrosion resistance of commercially pure titanium
[J].
Speciation of the H2SO4-Fe2(SO4)3-FeSO4-H2O system and development of an expression to predict the redox potential of the Fe3+/Fe2+ couple up to 150oC
[J].