随着石油天然气使用的日益增加,油气的储藏和运输变得至关重要。油库的建立则是为了方便油气的储运,在众多的运输途径中,海上运输是最为便利、高效和低成本的一种运输方式,因此我国油库大部分临海而建。通常情况下,沿海地区由于处在海洋大气环境中,Cl-浓度较高。对于湛江油库来说,其靠近沿海,不仅大气中Cl-含量高,周围环境湿度也大,年平均相对湿度可高达81.48%,还存在一定浓度的工业污染物SO2,且因位于北纬21°09′、东经110°24′,较靠近赤道导致紫外辐照强,油库处在高湿高辐照的海洋工业大气环境中。在湛江油库进行服役的油罐抗压环常用材料低碳钢Q235 (CS)、油气运输常用材料管线钢L415 (PS)和油罐外壁常用材料压力容器钢16MnNi (PVS)因直接暴露在大气中而极易发生腐蚀,因此研究3种材料在湛江大气环境下的腐蚀行为具有重要意义。Song和Chen[8]研究了紫外照射在NaCl诱导Q235碳钢大气腐蚀中的作用,结果表明,紫外照射通过影响具有半导体性质的腐蚀产物γ-FeOOH的光电效应来加速碳钢的腐蚀速率。Mao等[9]研究了Cl-的存在对管线钢在NaHCO3溶液中的钝化和腐蚀的影响,结果发现,管线钢在含Cl-的NaHCO3溶液中的腐蚀坑尺寸大于在无Cl-溶液的腐蚀坑尺寸。陈惠玲和魏雨[10]对碳钢在含有SO2大气中的腐蚀行为进行研究,发现SO2的存在会导致碳钢的腐蚀速率加快,并且在腐蚀初期表现最为明显。吕旺燕等[11]研究了NaHSO3和NaCl的协同作用对Q235腐蚀的影响,结果表明,Q235在NaHSO3溶液中会生成较多的α-FeOOH;在NaCl溶液中则生成较多的γ-FeOOH。同时还发现,Q235在2种腐蚀因素共同作用下的腐蚀速率高于单一腐蚀因素影响的腐蚀速率。由此可见,紫外照射、Cl-和SO2的存在都会加速钢材的腐蚀速率,进而影响其使用寿命。
尽管户外暴露实验周期长,但是可以获得真实可靠的信息。近年来,关于油库常用金属材料在高湿高辐照的海洋工业大气环境下的初期腐蚀行为研究较少。本工作以Q235、L415和16MnNi钢为研究对象,研究其暴露在湛江油库实际大气环境中180 d的初期腐蚀行为。通过失重分析、腐蚀产物分析、腐蚀产物形貌观察、电化学测试等手段对腐蚀样品进行分析,揭示3种材料在相同油库环境下的腐蚀行为差异。
1 实验方法
表1 Q235、L415和16MnNi钢的化学成分 (mass fraction / %)
Table 1
| Steel | C | Si | P | S | Cr | Mn | Ni | Fe |
|---|---|---|---|---|---|---|---|---|
| Q235 | 0.15 | 0.11 | 0.033 | 0.010 | 0.05 | 0.36 | 0.03 | Bal. |
| L415 | 0.08 | 0.16 | 0.015 | < 0.003 | - | 1.61 | - | Bal. |
| 16MnNi | 0.11 | 0.26 | 0.011 | < 0.003 | 0.03 | 1.32 | 0.03 | Bal. |
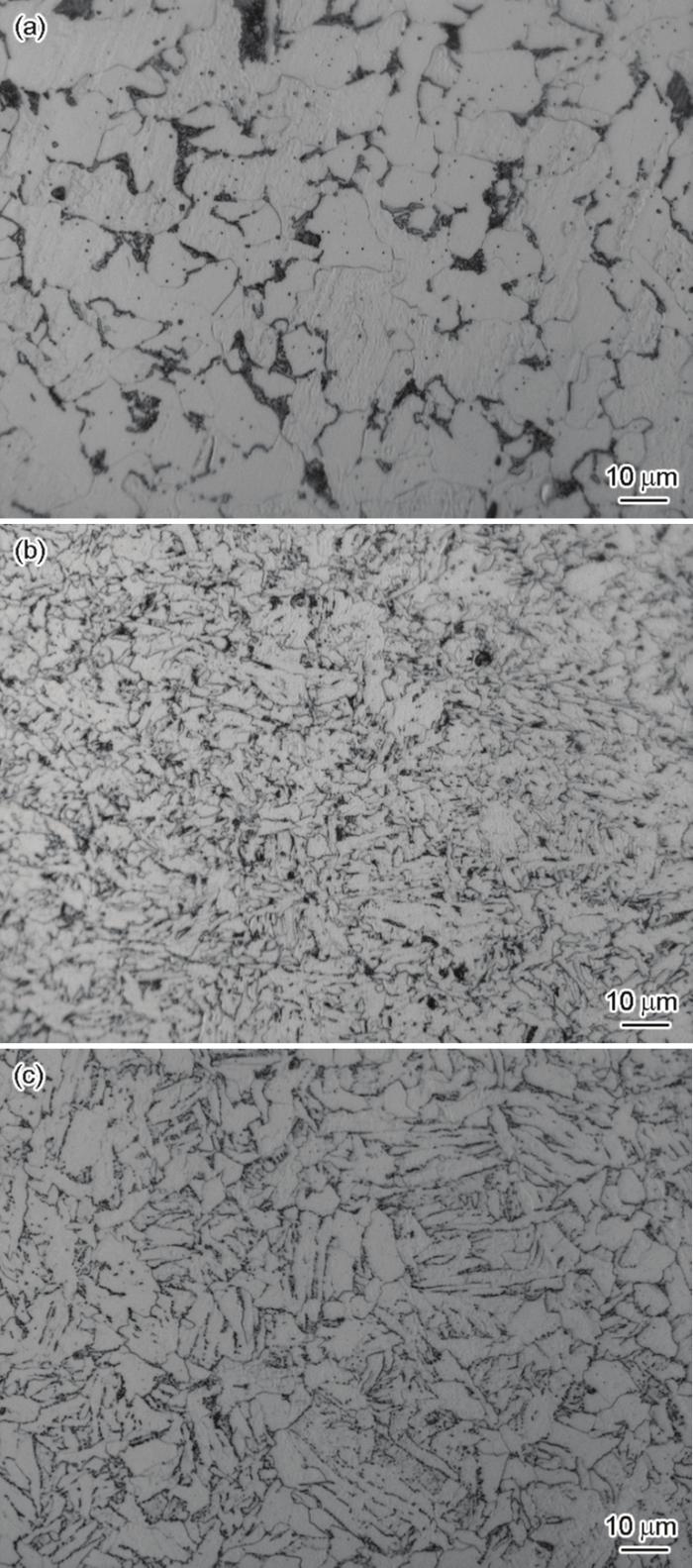
图1
图1
Q235、L415和16MnNi钢的微观组织
Fig.1
Microstructures of Q235 (a), L415 (b), and 16MnNi (c) steels
实验地点为湛江中石化厂区,空气中SO2的含量为0.0134 mg/(100 cm2·d),Cl-含量为0.0276 mg/(100 cm2·d)。样品固定在试样架上,朝向正南方向放置且与地面之间形成45°夹角。样品投放时间为2020年6月30日到2020年12月27日,实验周期为180 d。在此期间,湛江环境条件随时间的变化关系如表2所示。在7、8月份的时候,湛江温度最高。随着时间延长,日均温度降低,晴天天数增多。
表2 湛江大气环境随时间的变化
Table 2
| Time | Tmax | Tmin | Cloudy | Rain | Sun |
|---|---|---|---|---|---|
| month | oC | oC | d | d | d |
| 7 | 33 | 27 | 14 | 16 | 0 |
| 8 | 33 | 26 | 14 | 16 | 0 |
| 9 | 33 | 25 | 16 | 13 | 1 |
| 10 | 30 | 22 | 20 | 7 | 3 |
| 11 | 26 | 20 | 20 | 8 | 2 |
| 12 | 21 | 15 | 21 | 5 | 4 |
根据GB/T16545—2015标准配置除锈液(室温下,500 mL蒸馏水+ 500 mL浓盐酸+ 3.5 g六次甲基四胺),将去除表面锈层的腐蚀样品置于其中。用毛刷轻刷样品表面直到所有腐蚀产物都去掉,然后用蒸馏水和酒精快速清洗,干燥24 h后用电子天平称重。
用研磨钵对刮取下来的腐蚀产物进行研磨,通过D/Max-2500PC X射线衍射仪(XRD)对腐蚀粉末进行物相分析,Cu靶,扫描角度2θ = 10°~70°,扫描速率10°/min。在测试过程中,通过控制每个样品使用相同的粉末量,可以比较不同样品的腐蚀产物的强度差异。因此,腐蚀产物各相的峰值强度可以半定量地反映其相对含量[12]。利用Jade软件对XRD结果进行标定,并在Origin中进行制图。使用ESEM XL30 FEG环境扫描电子显微镜(SEM)观察腐蚀样品的表面形貌和截面形貌。在实验前,为了增强样品的导电性,对样品表面进行喷碳处理。
利用PARSTAT 2273电化学工作站对腐蚀样品进行电化学阻抗谱(EIS)和动电位极化测量。电化学测试采用经典的三电极体系,腐蚀样品用松香石蜡封好后作为工作电极,工作面积为1 cm2,饱和甘汞电极(SCE)和干净的Pt片分别作为参比电极和对电极,电解液为0.1 mol/L的Na2SO4溶液,扫描速率为0.33 mV/s。EIS测量时,频率范围为105~10-2 Hz。待开路电位(OCP)稳定后(1 min之内,开路电位的变化不超过1 mV),开始进行电化学测试。
2 实验结果
2.1 失重分析
式中,wo为实验前样品质量,g;ws为实验后样品质量,g;ρ为密度,7.86 g/cm3;S为样品的工作面积,cm2。
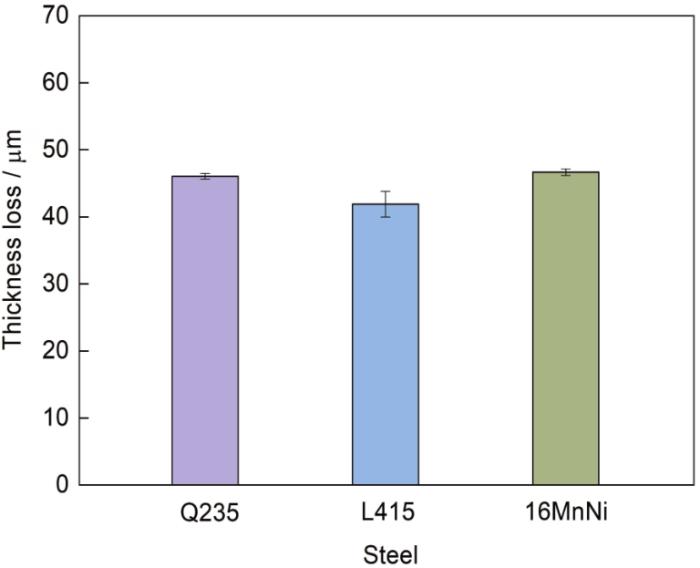
图2
图2
Q235、L415和16MnNi钢在湛江大气环境中暴露180 d时的厚度损失
Fig.2
Thickness losses of Q235, L415, and 16MnNi steels exposed to Zhanjiang atmospheric environment for 180 d
式中,VD为平均腐蚀速率,μm/d;Dn 为厚度损失,μm;t为腐蚀时间,d;n为试样周期(n = 1,对应腐蚀时间为180 d)。由于此次实验只有一个取样周期,所以厚度损失可以清晰直接地反映平均腐蚀速率,3种材料Q235、L415、16MnNi的平均腐蚀速率分别为0.256、0.233和0.248 μm/d,由大到小依次为:Q235、16MnNi和L415。
2.2 腐蚀形貌分析
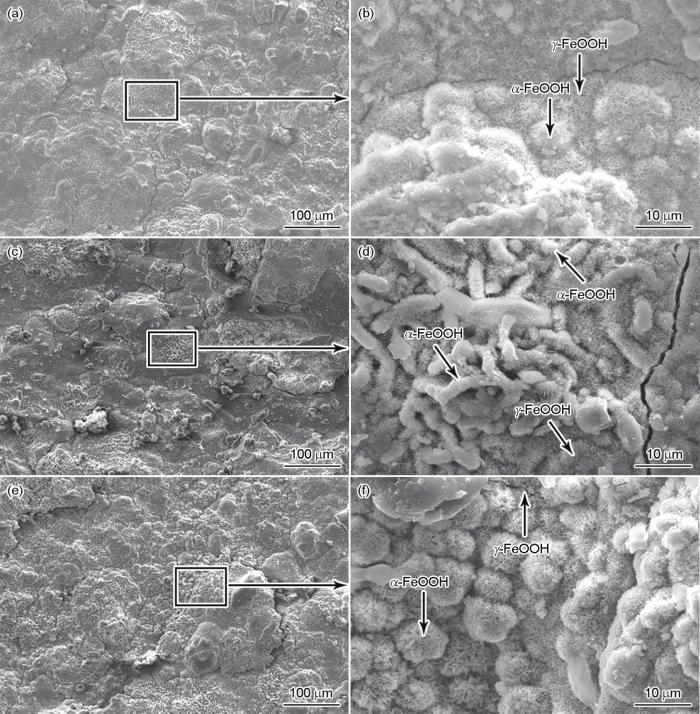
图3为Q235、L415和16MnNi暴露在湛江大气环境中180 d时的表面腐蚀形貌。可以看出,3种材料的基体表面都形成了一定厚度的锈层。Q235在第180 d时锈层表面出现了大量的微裂纹(图3a),同时还生成了针状的γ-FeOOH和棉球状的α-FeOOH[16] (图3b)。L415锈层表面平坦,孔隙率低,局部锈层致密(图3c),靠近基体的位置生成了较多的针状的γ-FeOOH,远离基体处出现了较多的棒状的α-FeOOH[17] (图3d),这一现象表明棉球状的α-FeOOH已经部分长成为棒状的α-FeOOH。从图3f可以看出,16MnNi锈层上也布满了大量的棉球状的α- FeOOH和少量的针状的γ-FeOOH。尽管α-FeOOH可以抑制腐蚀过程的发生,但微裂纹和γ-FeOOH的存在均有利于腐蚀过程的进行。
图3
图3
Q235、L415和16MnNi钢在相同时间相同环境下的表面形貌(180 d)
Fig.3
Low (a, c, e) and locally high (b, d, f) magnified surface morphologies of Q235 (a, b), L415 (c, d), and 16MnNi (e, f) steels at the same time and the same environment for 180 d
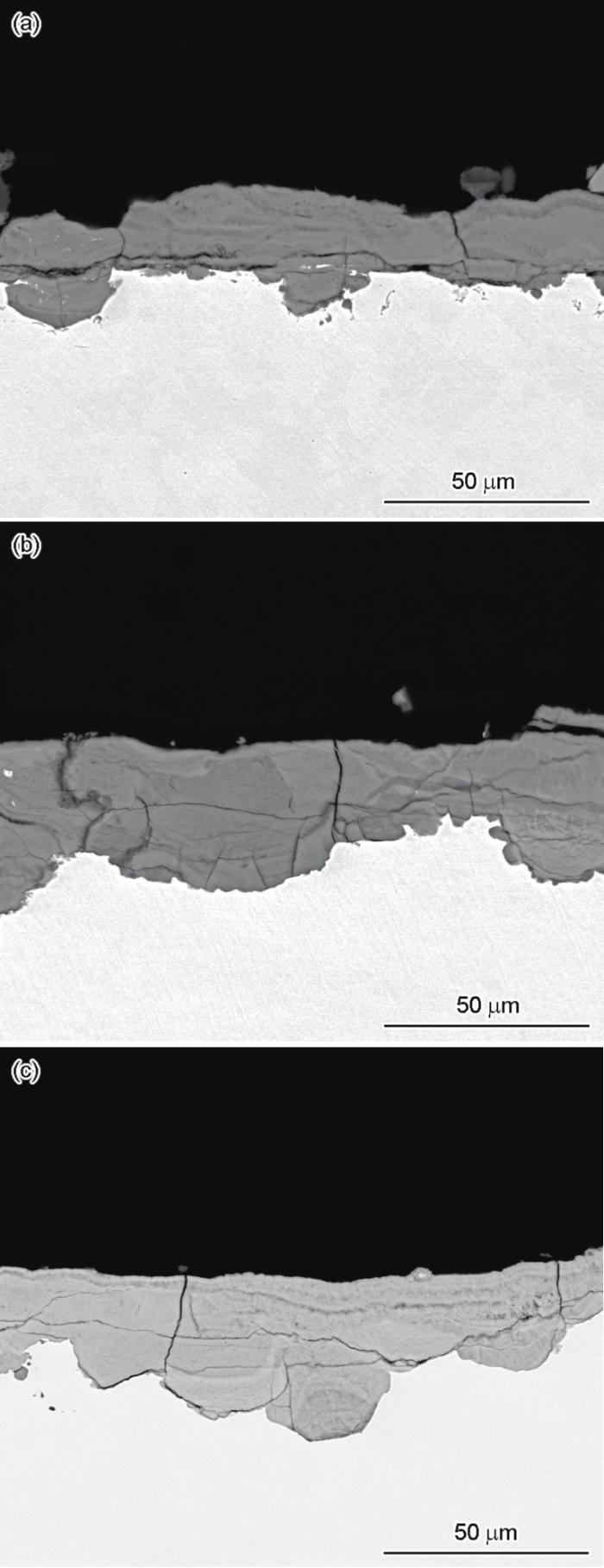
图4
图4
Q235、L415和16MnNi钢暴露在湛江大气环境中180 d时的截面形貌
Fig.4
Cross-section morphologies of Q235 (a), L415 (b), and 16MnNi (c) exposed to Zhanjiang atmospheric environment for 180 d
如此循环进行,酸会不断溶解锈层中较为活泼的物质,导致锈层内部含有微小孔洞。随着时间的延长,锈层中的孔洞半径逐渐增加,沿着横纵方向不断扩展,孔洞之间结合起来,既可以发展为横向裂纹,也可以形成纵向裂纹。对比3种材料的截面厚度可以看出,Q235锈层厚度最小,但其厚度损失最大,L415和16MnNi锈层较厚,厚度损失反而较小。这是因为Q235锈层内部有着尺寸较大的横向裂纹,较大的横向裂纹会导致锈层之间结合力较差,当外锈层疏松时,横向裂纹的存在会加速锈层的脱落,导致锈层厚度减薄,此时Q235锈层厚度最小可能是与内锈层结合力较差的外锈层的脱落造成的,因此单纯通过样品锈层厚度比较样品厚度损失的大小是不太准确的。从截面结构可以看出,Q235的内锈层的致密度高于外锈层。16MnNi也有着明显的内外锈层结构,但是相比于Q235来说,其内锈层更加致密。而在L415的锈层中,外部锈层的致密性要高于内部锈层。上述3种材料的锈层中均有明显的分层结构,但内外锈层的致密程度不一致。尽管锈层结构对钢材后续的腐蚀过程会造成一定程度的影响,但是锈层结构不能较好地解释此环境下样品发生的腐蚀差异。
2.3 腐蚀产物分析
图5为Q235、L415和16MnNi在湛江油库服役180 d后腐蚀产物的XRD谱。可以看出,Q235、L415和16MnNi锈层中均含有α-FeOOH、γ-FeOOH和Fe3O4。其中只有Q235锈层中含有少量的β-FeOOH。Yamashita等[23]研究发现,Cl-的存在是β-FeOOH产生的前提,当基体表面的Cl-达到一定浓度时,就开始生成β-FeOOH。L415和16MnNi锈层中没有检测到β-FeOOH则是由于XRD技术的局限性,当锈层中β-FeOOH的含量低于2%时,XRD技术检测不到该产物。β-FeOOH的存在会促使锈层体积膨胀,导致裂纹的形成、锈层的开裂和剥落[24]。Asami和Kikuchi[25,26]还发现当β-FeOOH存在于锈层中时,锈层的孔隙度高于α-FeOOH和γ-FeOOH存在的锈层,这说明含有β-FeOOH的锈层更易捕捉大气中的Cl-,并作为Cl-反应的通道,有利于腐蚀介质的进入。因此,Q235锈层中较多的β-FeOOH的存在导致了其粗糙多孔的锈层结构。
图5
图5
Q235、L415和16MnNi钢暴露在相同环境相同时间下的XRD谱
Fig.5
XRD spectra of powdered rust Q235, L415, and 16MnNi steels exposed to the same environment and the same time for 180 d
除了β-FeOOH不同外,Q235、L415和16MnNi锈层中其他腐蚀产物的含量也存在一定差异。如可以加速腐蚀过程的γ-FeOOH和Fe3O4在3种材料锈层中的含量排序均是Q235 > 16MnNi > L415,而α-FeOOH在3种材料锈层中的含量相差不大。由此可以推断出,在α-FeOOH含量一定的情况下,β-FeOOH、γ-FeOOH和Fe3O4的相对含量直接影响钢材的腐蚀速率。α-FeOOH作为一种电绝缘体[8],几乎不受紫外照射的影响,其在含有
2.4 电化学
图6
图6
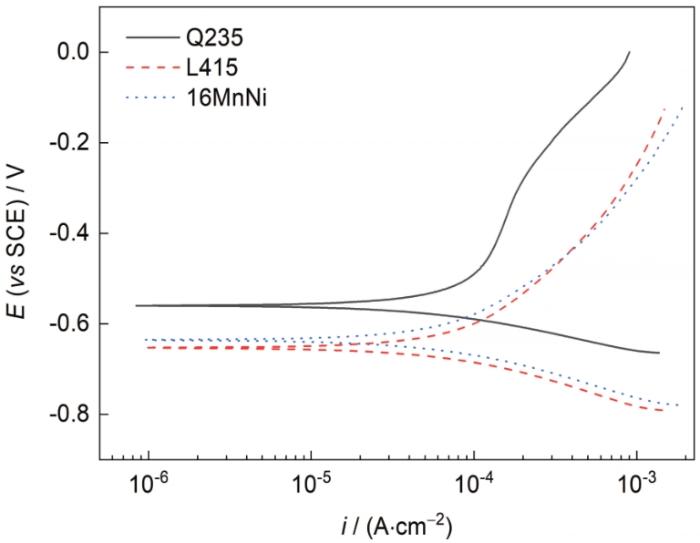
Q235、L415和16MnNi钢暴露在湛江大气环境中180 d的极化曲线
Fig.6
Potentiodynamic polarization curves of Q235, L415, and 16MnNi steels exposed to Zhanjiang atmospheric environment for 180 d (E—potential, i—current density)
表3 Q235、L415和16MnNi钢的腐蚀电位(Ecorr)和腐蚀电流密度(icorr)
Table 3
| Steel | Ecorr / mV | icorr / (μA·cm-2) |
|---|---|---|
| Q235 | -559.5 | 107.20 |
| L415 | -662.9 | 77.82 |
| 16MnNi | -652.1 | 86.79 |
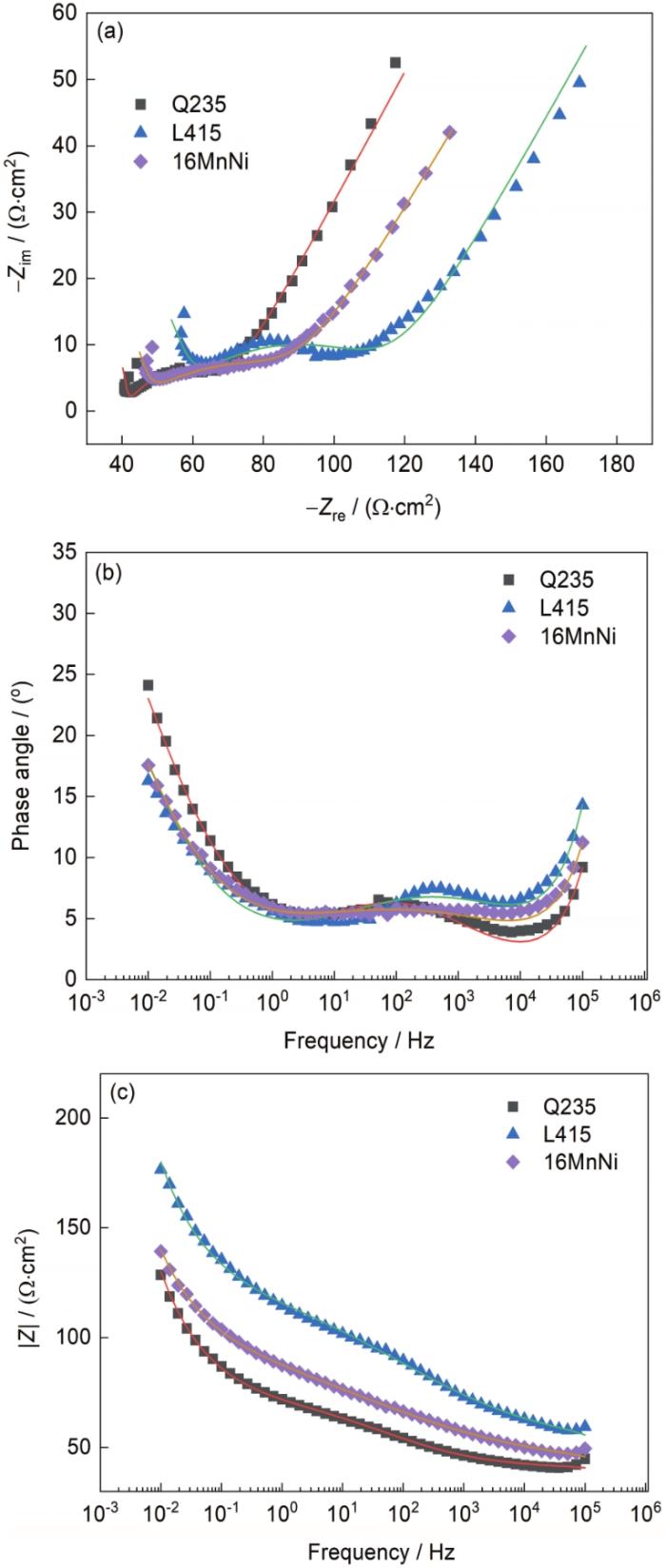
图7为Q235、L415和16MnNi在相同时间、相同环境下的Nyquist和Bode图。从Nyquist图中可以看出,3种材料在低频区出现了Warburg阻抗,说明样品表面发生了扩散控制的腐蚀过程。图8为利用3种材料的EIS数据进行拟合得到的等效电路图。其中Rs为溶液电阻,Rr为锈层电阻,Rct为电荷转移电阻,Qr为锈层电容,Qdl为双层电容,Zw为Warburg扩散阻抗,该阻抗与腐蚀介质通过锈层中的孔隙向基体表面扩散有关[27]。表4为EIS的电路拟合参数结果,拟合误差均处在10-4数量级,这表明电化学数据拟合结果较好。通常情况下,Rct的值可以较好地反映锈层的保护能力,即Rct与腐蚀速率成反比[32,33]。通过表4可以看出,3种材料Rct的大小顺序为:Rct(Q235) < Rct (16Mn) < Rct (L415),这表明此时L415锈层较为致密,对基体的保护能力最好,而Q235锈层的保护能力最差。
图7
图7
Q235、L415和16MnNi钢暴露在湛江大气环境下180 d的Nyquist图和Bode图
Fig.7
Nyquist (a) and Bode (b, c) diagrams of Q235,L415, and 16MnNi steels exposed to Zhanjiang atmospheric environment for 180 d
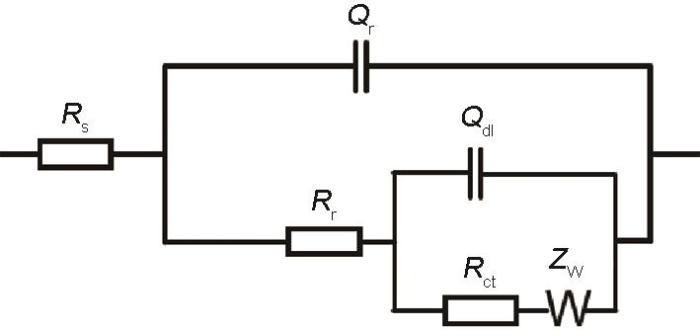
图8
图8
EIS的等效电路
Fig.8
Equivalent circuit of EIS (Rs—the electrolyte resistance, Rr—the rust layer resistance, Rct—the charge transfer resistance, Qr—the rust layer capacitance, Qdl—the double layer capacitance, Zw—the barrier diffusion impedance)
表4 等效电路的拟合参数
Table 4
| Steel | Rs / (10-4 Ω·cm2) | Qr / (10-9 F·cm-2) | nr | Rr / (Ω·cm2) | Qdl / (10-3 F·cm-2) | ndl | Rct / (Ω·cm2) | Zw / (10-2 Ω·cm2) | χ2 / 10-4 |
|---|---|---|---|---|---|---|---|---|---|
| Q235 | 2.674 | 5.617 | 1 | 40.47 | 2.216 | 0.4563 | 31.39 | 5.296 | 3.98 |
| L415 | 106.7 | 5.602 | 1 | 51.55 | 1.588 | 0.3364 | 72.15 | 4.795 | 3.68 |
| 16MnNi | 13.68 | 5.160 | 1 | 41.46 | 1.134 | 0.2762 | 61.60 | 5.365 | 2.61 |
2.5 腐蚀机理
湛江湿度大,当大气环境中的湿度达到一定值时,Q235、L415和16MnNi基体就可以吸附周围环境中的水蒸气从而在表面形成一层薄液膜,使环境中的O2、Cl-和SO2溶解在其中,与基体发生一系列的电化学反应。
① 当薄液膜中的O2充足时,Fe可以被氧化成FeOH+,进而转变成γ-FeOOH。随着时间增加,锈层增厚,锈层中O2含量降低,Fe3O4在O2不充足的环境下可以转换成α-FeOOH (
3 结论
(1) 成分不同的Q235、L415和16MnNi在高湿高辐照的湛江大气环境中服役相同时间时,形成的主要腐蚀产物种类相同,均含有α-FeOOH、γ-FeOOH和Fe3O4,只是锈层中的γ-FeOOH和Fe3O4的含量有明显区别。Q235因其粗糙多孔的锈层结构和锈层较多的β-FeOOH、γ-FeOOH和Fe3O4而导致腐蚀速率最快。
(2) 锈层成分显著影响钢材的腐蚀过程,Q235、L415和16MnNi在高湿高辐照的海洋工业大气环境下发生的腐蚀差异主要是由于腐蚀产物中β-FeOOH、γ-FeOOH和Fe3O4的含量不同造成的。
(3) Cl-、SO2和紫外辐照3者的协同作用,促使Q235、L415和16MnNi锈层内部分布着长短不一的横纵裂纹和微小孔洞,破坏了锈层的完整性,使腐蚀性介质可以通过锈层与基体直接反应,进一步加剧腐蚀。
参考文献
Characterisation of initial atmospheric corrosion carbon steels by field exposure and laboratory simulation
[J].
Effect of relative humidity on corrosion of Q235 carbon steel under thin electrolyte layer in simulated marine atmosphere
[J].The purpose of this paper is to obtain the environmental factor, which has the greatest effect on the corrosion rate of Q235 carbon steel under thin electrolyte layer, and to analyze the effect of this factor on the corrosion morphology, corrosion products and polarization process of Q235 carbon steel.
Corrosion behavior of Q235 in simulated natural environment by electrochemical technology
[J].
Q235钢在模拟自然环境下失效行为的电化学研究
[J].
An electrochemical study of phase-transitions in rust layers
[J].
Atmospheric corrosion of carbon steel in Colombia
[J].
Influence of environmental factors on atmospheric corrosion in dynamic environment
[J].
Corrosion exposure study on Q235 steel in marine atmospheric
[J].
Q235钢海洋大气腐蚀暴露试验研究
[J].
The role of UV illumination on the NaCl-induced atmospheric corrosion of Q235 carbon steel
[J].
Pitting corrosion of pipeline steel in dilute bicarbonate solution with chloride ions
[J].
Mechanism of industrial atmospheric corrosion for carbon steel
[J].
碳钢在含SO2环境大气中腐蚀机理的研究
[J].
Synergic effect of NaHSO3 and NaCl on atmospheric corrosion of Q235 steel
[J].
NaHSO3和NaCl对Q235钢大气腐蚀的协同作用
[J].
Composition and protective ability of rust layer formed on weathering steel exposed to various environments
[J].
Outdoor atmospheric corrosion of carbon steel and weathering steel exposed to the tropical-coastal climate of Thailand
[J].
Initial atmospheric corrosion of carbon steel in industrial environment
[J].
The effect of β-FeOOH on the corrosion behavior of low carbon steel exposed in tropic marine environment
[J].
Corrosion of low carbon steel in atmospheric environments of different chloride content
[J].
Corrosion behaviors of low alloy steel and carbon steel deposited with NaCl and NaHSO3 under dry/humid alternative condition
[J].
表面沉积NaCl和NaHSO3的低合金钢和碳钢在干湿交替条件下的腐蚀行为
[J].利用Fourier变换红外(FTIR)光谱、XRD和SEM技术以及恒电流测试技术, 研究了表面沉积NaCl和NaHSO<sub>3</sub>的低合金钢和碳钢在干湿交替条件下的腐蚀行为. 结果表明, 低合金钢和碳钢的腐蚀速率都随着实验周期的延长而减小, 且其失重量基本相同, 低合金钢并未表现出比碳钢优越的耐蚀性. 主要腐蚀产物为α-FeOOH, Fe<sub>3</sub>O<sub>4</sub>, γ-FeOOH和硫酸盐, 此外还存在大量的δ-FeOOH, 导致腐蚀速率降低.
Evolution of corrosion of MnCuP weathering steel submitted to wet/dry cyclic tests in a simulated coastal atmosphere
[J].
Evolution of atmospheric corrosion of MnCuP weathering steel in a simulated coastal-industrial atmosphere
[J].
Effect of deposition of NaCl on the initial atmospheric corrosion of Q235
[J].
NaCl颗粒沉积对Q235钢早期大气腐蚀的影响
[J].
Rusting evolution of MnCuP weathering steel submitted to simulated industrial atmospheric corrosion
[J].
A study on the initial corrosion behavior of carbon steel exposed to a simulated coastal-industrial atmosphere
[J].Carbon steels as common structural material have been widely used for basic facilities with the development of the city. In these service environments, carbon steel would inevitably encounter the atmospheric corrosion. Especially, the corrosion of carbon steels exposed to coastal-industrial atmosphere is very outstanding. However, the initial corrosion mechanism of carbon steel subjected to coastal-industrial environment still need to be clarified, which would be vital for predicating the subsequent corrosion process. In addition, although many scholars studied the synergism of SO2 and Cl-, which obviously accelerates the corrosion of steel and reduces its service life, there is few research about the effect of the synergism of SO2 and Cl- (in different proportion) on the early corrosion behavior of the carbon steel. Therefore, it is of great importance to investigate the initial corrosion mechanism of carbon steel and the effect of the synergism of SO2 and Cl- (in different proportion) in the coastal-industrial atmosphere. In present work, the initial corrosion behavior of Q235 carbon steel exposed to a simulated coastal-industrial atmosphere has been studied by weight loss, XRD, SEM and electrochemical measurements. Also, the effect of the synergism of SO2 and Cl- (in different proportion) on the early corrosion behavior of Q235 car bon steel has been investigated. The results indicate that the initial corrosion behavior of carbon steel exposed to a simulated coastal-industrial atmosphere presented a transition from corrosion acceleration to deceleration, and the kinetics of accelerated corrosion process followed the empirical equation D=Atn. A double-layered corrosion product was formed on the surface of carbon steel after 24 h: the loose outer layer and relative dense inner layer; the synergistic effect between SO2 and Cl- accelerated the corrosion of carbon steel. However, the change in the ratio of SO2 and Cl- had no significant effect on the corrosion loss of carbon steel, and had not changed the composition of corrosion products formed on carbon steel surface. SO2 caused the corrosion morphology of carbon steel to tend to uniform corrosion.
碳钢在模拟海洋工业大气环境中初期腐蚀行为研究
[J].采用失重分析、X射线衍射分析、扫描电镜分析及电化学测试分析方法对Q235碳钢在模拟海洋工业大气环境中的初期腐蚀历程和机理开展深入研究,并着重探究了不同比例SO<sub>2</sub>和Cl<sup>-</sup>的协同效应对碳钢初期腐蚀行为机制的影响。结果表明,Q235碳钢在模拟海洋工业大气环境中的初期腐蚀呈现由加速过程向减速过程转化的特点,且加速过程的腐蚀动力学仍遵循幂函数规律D=At<sup>n</sup>;腐蚀24 h后,腐蚀产物呈现双层结构,即疏松的外层和相对致密的内层。SO<sub>2</sub>和Cl<sup>-</sup>的协同效应会加速碳钢的腐蚀,但二者比例的变化对碳钢腐蚀失重影响并不明显,也没有改变腐蚀产物成分,SO<sub>2</sub>促使碳钢腐蚀形态趋向于均匀腐蚀。
In situ observation of initial rust formation process on carbon steel under Na2SO4 and NaCl solution films with wet/dry cycles using synchrotron radiation X-rays
[J].
On the akaganéite crystal structure, phase transformations and possible role in post-excavational corrosion of iron artifacts
[J].
In-depth distribution of rusts on a plain carbon steel and weathering steels exposed to coastal-industrial atmosphere for 17 years
[J].
Characterization of rust layers on weathering steels air-exposed for a long period
[J].
Effect of sulphur dioxide on the corrosion of a low alloy steel in simulated coastal industrial atmosphere
[J].
Electrochemical study of indoor atmospheric corrosion layers formed on ancient iron artefacts
[J].
Airborne chloride deposit and its effect on marine atmospheric corrosion of mild steel
[J].
Atmospheric corrosion analysis and rust evolution research of Q235 carbon steel at different exposure stages in Chengdu atmospheric environment of China
[J].
In situ Raman spectroscopy study of corrosion products on the surface of carbon steel in solution containing Cl- and
Effect of Mn additions on the corrosion behaviour of TWIP Fe-Mn-Al-Si austenitic steel in chloride solution
[J].Corrosion of TWIP austenitic steels Fe-Mn-3AI-3Si was studied using polarization curves, EIS tests and XPS. Results indicated that increased concentrations of Mn were detrimental to its corrosion resistance. XPS study revealed that pseudo-protective oxide layer was composed of FeO, Fe2O3, FeOOH, Al2O3, MnO and MnO2. Enrichment of Mn oxides in the outermost oxide layer was found as Mn increased in the TWIP steel bulk along with a decrease in Fe oxides. The increased corrosion susceptibility exhibited with the concentration of Mn in the alloys was associated with the greater contribution of the less protective Mn oxide in the surface film.
EIS monitoring study of atmospheric corrosion under variable relative humidity
[J].
Study of corrosion evolution of carbon steel exposed to an industrial atmosphere
[J].
The corrosion mechanisms of carbon steel and weathering steel in SO2 polluted atmospheres
[J].