HEA因Cr含量超过12% (原子分数)可发生钝化,钝化行为是HEA腐蚀研究的重要课题。Wang等[5]发现,Co36Fe36Cr18Ni10 (原子分数,%,下同) HEA在模拟人体体液中的钝化膜的Cr2O3 / Cr(OH)3比值高于316L不锈钢;Xu等[6]指出,激光熔凝制备的CoCrFeMnNi HEA的钝化膜具有较高的(Cr + Ni + Co) / (Fe + Mn)的原子比,其钝化膜保护能力更强;Luo等[7]发现,CoCrFeMnNi HEA的钝化膜中存在Ni元素,并指出这与不锈钢钝化膜组成有一定区别。然而,关于AlCoCrFeNi2.1 EHEA钝化行为的研究鲜有报道。局部腐蚀是HEA关注的另一重点问题。Shi等[8]发现,Al元素的增加引起Alx CoCrFeNi HEA中贫Cr的bcc相的体积分数增大,导致钝化膜致密性下降进而诱发局部腐蚀。Chai等[9]认为,多主元合金FeCoNiCrx 中过量的Cr引起的元素偏析是诱发局部腐蚀的原因。Lin和Tsai[10]认为,Al0.5CoCrFeNi HEA中的富Al-Ni相在含Cl-的环境中优先腐蚀。此外,AlCoCrFeNi2.1 EHEA由fcc相与B2相组成,两相之间可能形成微电偶效应,存在局部腐蚀的风险。
因此,本工作采用循环极化曲线、电化学交流阻抗谱(EIS)、Mott-Schottky (M-S)曲线、扫描电子显微镜(SEM)和X射线光电子能谱(XPS)等方法研究AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的电化学特征、钝化行为与局部腐蚀规律,并揭示其微观腐蚀机理。
1 实验方法
1.1 实验材料与测试溶液
所用AlCoCrFeNi2.1 EHEA是以纯度为99.9% (质量分数,下同)的Al、Co、Ni与纯度为99.5%~99.6%的Cr、Fe为原料,真空电弧熔炼而成。AlCoCrFeNi2.1 EHEA经电火花线切割加工后,使用SiC砂纸由250~5000号逐级打磨,利用2.5 μm金刚石研磨膏机械抛光,经王水(HCl∶HNO3 = 3∶1,体积比)侵蚀后在M33OP-HD228S光学显微镜(OM)下观察组织形貌。实验使用的测试溶液为0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液。
1.2 电化学测试
用于电化学测试的AlCoCrFeNi2.1 EHEA试样尺寸为10 mm × 10 mm × 2 mm。电化学试样制作方法为将铜导线与试样进行焊接,使用环氧树脂封装,露出10 mm × 10 mm的测试面,试样使用SiC砂纸逐级打磨至2000号,用去离子水、无水乙醇清洗后冷风吹干。电化学测试均在CS350电化学工作站上完成,采用传统的三电极系统,以饱和甘汞电极(SCE)作为参比电极,Pt电极作为对电极,AlCoCrFeNi2.1 EHEA作为工作电极。电化学测试前,样品在测试溶液中浸泡1 h以保证试样表面达到相对稳定的状态。EIS测试频率范围为104~10-2 Hz,振动电位为10 mV,EIS数据利用ZsimpWin软件拟合分析。循环极化曲线测试从-1.0 V (vs SCE)开始扫描,当电流密度达到1 mA/cm2时进行回扫,扫描速率为0.5 mV/s。为研究AlCoCrFeNi2.1 EHEA试样的钝化行为,对其进行恒电位极化2 h处理,参照极化曲线测试结果,电位选择为0.1和 0.2 V (vs SCE),随后进行M-S曲线测试,测试范围为-1.0~1.0 V (vs SCE),频率为103 Hz。
1.3 XPS测试
为研究钝化膜性质,对AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中进行0.2 V (vs SCE)恒电位极化2 h以在金属表面形成钝化膜。然后,应用具有单色Al Kα X射线源的AXIS SUPRA+型XPS对钝化膜的成分进行分析。数据使用标准C1s峰(284.8 eV)进行校准,并使用XPSPEAK 4.1软件进行数据拟合。
1.4 浸泡实验
将AlCoCrFeNi2.1 EHEA试样分别于0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中浸泡25 d,然后,采用配备能量色散光谱仪(EDS,Xplore 30)的CLARA型SEM进行腐蚀形貌观察与元素分布分析。
2 实验结果
2.1 AlCoCrFeNi2.1 EHEA的微观结构
图1
图1
AlCoCrFeNi2.1共晶高熵合金(EHEA)显微组织
Fig.1
Microstructure of AlCoCrFeNi2.1 eutectic high-entropy alloy (EHEA)
2.2 AlCoCrFeNi2.1 EHEA的循环极化曲线
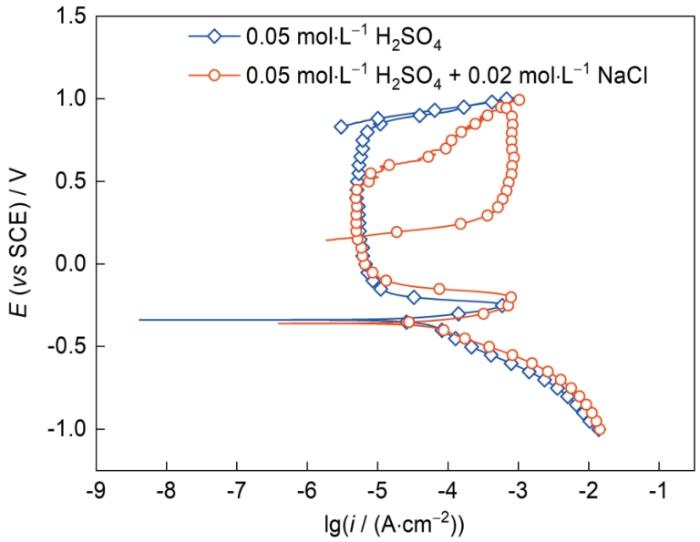
图2为AlCoCrFeNi2.1 EHEA分别在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的循环极化曲线,具体的电化学参数如表1所示。由图可知,AlCoCrFeNi2.1 EHEA在2种溶液中均表现出活化-钝化-过钝化的行为,这与Sun等[16]测得的CrMnFeNi与CrMnFeNiLa0.1合金在不同浓度H2SO4溶液中的动电位极化曲线的现象一致。AlCoCrFeNi2.1 EHEA在2种溶液中自腐蚀电位(Ecorr)差异不大,这表明腐蚀的热力学倾向受Cl-影响不大;极化曲线的阳极区受到Cl-影响较大,钝化区明显变窄,破钝电位(Eb)下降,表明钝化膜在含Cl-的H2SO4溶液中更容易遭到破坏。值得注意的是,维钝电流密度(ipass)未发生明显变化,表明少量的Cl-并未改变钝化膜形成的势垒;此外,Cl-对阴极反应有轻微促进作用。循环极化曲线常用于研究钝化金属点蚀行为的电化学机理。当曲线的回扫电流密度比正向扫描的更大时会出现回滞环,回滞环的面积越大表明点蚀倾向越大;反之则表明合金具有较好的耐点蚀性能。从图2可知,0.05 mol/L H2SO4溶液中的循环极化曲线未出现回滞环,而在0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中则出现明显的回滞环,这表明AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中更容易发生点蚀。回滞环与阳极极化曲线的交点称作保护电位(Eprot),在Eprot之下,已有的点蚀不会继续生长;而在Eb与Eprot之间,已有的点蚀会继续生长但不会出现新的点蚀,Eprot越高表明合金的耐点蚀性能越优异[17~19]。少量的Cl-导致Eprot明显降低,耐点蚀性能下降,间接表明少量Cl-对AlCoCrFeNi2.1 EHEA钝化膜破坏作用明显。
图2
图2
AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的循环极化曲线
Fig.2
Cyclic polarization curves of AlCoCrFeNi2.1 EHEA in 0.05 mol/L H2SO4 solution and 0.05 mol/L H2SO4 + 0.02 mol/L NaCl solution (E—potential, i—current density)
表1 AlCoCrFeNi2.1 EHEA的电化学参数
Table 1
| Solution | Ecorr / mV | icorr / (μA·cm-2) | Eb / mV | ipass / (μA·cm-2) |
|---|---|---|---|---|
| 0.05 mol·L-1 H2SO4 | -339 | 59.012 | 778 | 4.990 |
| 0.05 mol·L-1 H2SO4 + 0.02 mol·L-1 NaCl | -360 | 67.387 | 471 | 5.048 |
2.3 AlCoCrFeNi2.1 EHEA的EIS
为进一步探讨AlCoCrFeNi2.1 EHEA的表面电化学行为,测试了其在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的EIS,如图3所示。AlCoCrFeNi2.1 EHEA在2种溶液中的Nyquist图(图3a)均由1个容抗弧及电感回路组成。H
图3
图3
AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的EIS
Fig.3
Nyquist plots (a) and Bode plots (b) of AlCoCrFeNi2.1 EHEA in 0.05 mol/L H2SO4 solut-ion and 0.05 mol/L H2SO4 + 0.02 mol/L NaCl solution (Inset in Fig.3a shows the electrical equivalent circuit of EIS. Rs—solution resistance, Qdl—electrochemical response parameter of double electric layers, Rct—the charge transfer resistance, L—inductance of adsorption species, RL—inductance resistance)
表2 AlCoCrFeNi2.1 EHEA的EIS拟合参数
Table 2
| Solution | Rs / (Ω·cm2) | Qdl / (Ω-1·cm-2·s n ) | n | Rct / (Ω·cm2) | L / (H·cm2) | RL / (Ω·cm2) |
|---|---|---|---|---|---|---|
| 0.05 mol·L-1 H2SO4 | 13.00 | 9.8442 × 10-5 | 0.8739 | 308.5 | 120.8 | 67.22 |
| 0.05 mol·L-1 H2SO4 + 0.02 mol·L-1 NaCl | 17.62 | 1.6432 × 10-4 | 0.8858 | 271.3 | 18.0 | 37.70 |
2.4 AlCoCrFeNi2.1EHEA的恒电位极化曲线
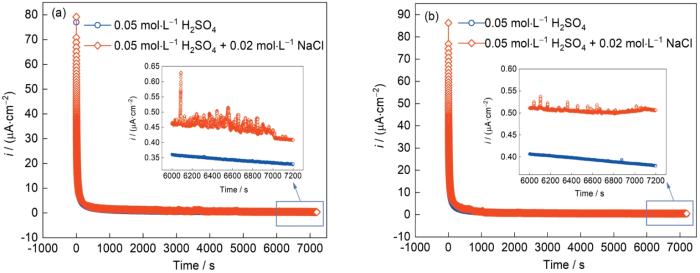
图4为AlCoCrFeNi2.1 EHEA分别在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的恒电位极化曲线。从图可知,随着极化的进行,电流密度在初步阶段急剧下降后保持在一个相对稳定的数值(iss),这表明钝化膜在合金表面不断形成并逐渐致密、稳定[27,28]。0.05 mol/L H2SO4溶液的极化曲线并未出现明显波动,而0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液的极化曲线波动较大,这表明少量的Cl-对钝化膜的形成有不利影响。2种极化电位下0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液的极化曲线中的iss均比0.05 mol/L H2SO4溶液的更大。有研究[29]表明,iss越大钝化膜的保护性能越差。
图4
图4
AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的恒电位极化曲线
Fig.4
Potentiostatic polarization curves of AlCoCrFeNi2.1 EHEA in 0.05 mol/L H2SO4 solution and 0.05 mol/L H2SO4 + 0.02 mol/L NaCl solution at 0.1 V (vs SCE) (a) and 0.2 V (vs SCE) (b) (Insets show the partial enlargement of potentiostatic polarization curves)
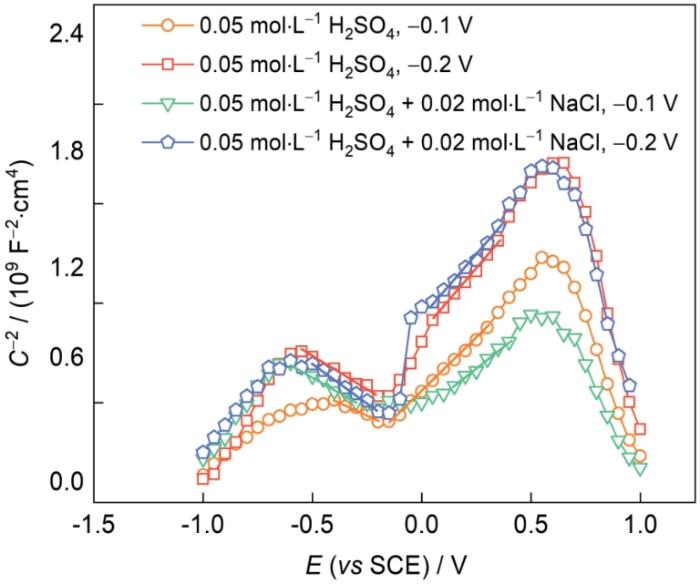
2.5 AlCoCrFeNi2.1EHEA的M-S曲线
图5
图5
AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的M-S曲线
Fig.5
Mott-Schottky (M-S) plots for AlCoCrFeNi2.1 EHEA in 0.05 mol/L H2SO4 solution and 0.05 mol/L H2SO4 + 0.02 mol/L NaCl solution (C-2—space charge capacitance)
式中,e为电子电荷,1.602 × 10-19 C;ε为钝化膜的介电常数,一般为15.6;ε0为真空介电常数,8.854 × 10-14 F/cm;k为Boltzmann常数,1.38 × 10-23 J/K;Efb和T分别为平带电位和热力学温度。
由图5可知,AlCoCrFeNi2.1 EHEA在不同电位不同溶液中的M-S曲线线型相似,均可分成2个区域,其中正斜率部分表示钝化膜呈现n型半导体性质,表明钝化膜中的主要缺陷为阳离子间隙或氧空位;负斜率部分表示钝化膜呈现p型半导体性质,表明钝化膜中主要缺陷为阳离子空位。通常,n型半导体与p型半导体分别对应钝化膜的内层与外层[30]。相对应的载流子密度(N)分别为施主密度(ND)和受主密度(NA),ND和NA可通过M-S曲线的线性区域斜率与
表3 AlCoCrFeNi2.1 EHEA以不同电位恒电位极化2 h后的受主密度和施主密度
Table 3
| Solution | E / V | NA / (1022 cm-3) | ND / (1021 cm-3) | Efb / V |
|---|---|---|---|---|
| 0.05 mol·L-1 H2SO4 | 0.1 | 1.8 | 8.6 | -0.53 |
| 0.2 | 1.5 | 6.9 | -0.65 | |
| 0.05 mol·L-1 H2SO4 + 0.02 mol·L-1 NaCl | 0.1 | 1.4 | 10.0 | -0.50 |
| 0.2 | 1.1 | 7.4 | -0.77 |
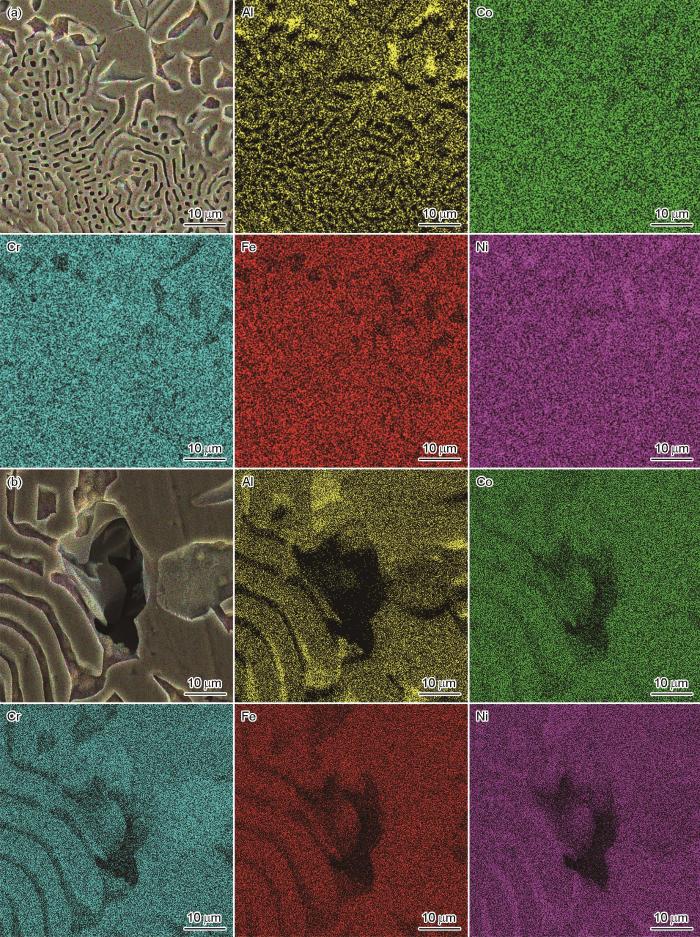
2.6 AlCoCrFeNi2.1EHEA浸泡后的形貌及成分
图6
图6
AlCoCrFeNi2.1 EHEA浸泡在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液25 d后的SEM像
Fig.6
SEM images of AlCoCrFeNi2.1 EHEA after immersion in 0.05 mol/L H2SO4 solution (a) and 0.05 mol/L H2SO4 + 0.02 mol/L NaCl solution (b) for 25 d
为进一步研究AlCoCrFeNi2.1 EHEA在0.05 mol/LH2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的局部腐蚀行为,采用EDS对其腐蚀表面的元素分布进行分析,结果如图7所示。由图可知,点蚀与选择性溶解均发生在富Ni-Al的B2相,富Cr的fcc相并未观察到明显的腐蚀现象。这是由于富Ni-Al的B2相中高含量的Al导致在B2相上形成的钝化膜中Al2O3含量提高,使得钝化膜致密性下降,而富Cr的fcc相形成的钝化膜相对更致密,具有更好的保护性[8,22,35]。此外,Hasannaeimi和Mukherjee[36]研究了AlCoCrFeNi2.1 EHEA在1%NaCl溶液中的定点微电偶腐蚀,发现fcc相富Cr、Co和Fe,而B2相的Al和Ni含量高,在极化及非极化条件下B2相的优先溶解是开始腐蚀的主要位置,这与本工作结果一致。AlCoCrFeNi2.1 EHEA的fcc相和B2相之间存在局部微电偶,这也是诱发局部腐蚀的原因之一。有研究[37]表明,晶粒尺寸会影响金属的钝化行为,AlCoCrFeNi2.1 EHEA的晶粒尺寸差异也会导致局部钝化膜性质的区别,可能引起电偶作用。也有学者[23]认为,富Ni-Al相优先溶解是由于Al和Ni中的键合,这导致Al、Ni易与H2SO4溶液中的OH-和SO
图7
图7
AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中浸泡25 d的EDS元素面分布
Fig.7
EDS element mappings of AlCoCrFeNi2.1 EHEA after immersion in 0.05 mol/L H2SO4 solution (a) and 0.05 mol/L H2SO4 + 0.02 mol/L NaCl solution (b) for 25 d
3 分析讨论
3.1 AlCoCrFeNi2.1EHEA的钝化行为
图8为在0.05 mol/L H2SO4溶液(图8a1~e1)和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液(图8a2~e2)中AlCoCrFeNi2.1 EHEA钝化膜的Al2p、Co2p3/2、Cr2p3/2、Fe2p3/2和Ni2p3/2的XPS结果。从图8a1和8a2可知,AlCoCrFeNi2.1 EHEA在2种测试溶液中的Al2p均由金属态Al (Al0,71.8/71.7 eV)峰和Al2O3 (73.8/73.7 eV)峰组成。图8b1和b2显示,Co2p3/2可分为5个组分峰,分别是金属态Co (Co0,777.7/777.8 eV)、CoO (779.9/780 eV)、Co3O4 (781.3 eV)、Co(OH)2 (782.5/782.6 eV)及Co的卫星峰(
图8
图8
AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中恒电位0.2 V (vs SCE)极化2 h后的高分辨XPS图
Fig.8
High resolution XPS spectra of AlCoCrFeNi2.1 EHEA in 0.05 mol/L H2SO4 solution (a1-e1) and 0.05 mol/L H2SO4 + 0.02 mol/L NaCl solution (a2-e2) after potentiostatic polarization at 0.2 V (vs SCE) for 2 h (%—peak area percentage)
(a1, a2) Al2p (b1, b2) Co2p3/2 (c1, c2) Cr2p3/2 (d1, d2) Fe2p3/2 (e1, e2) Ni2p3/2
AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中形成表面钝化膜的Al2p、Co2p3/2、Cr2p3/2、Fe2p3/2和Ni2p3/2各组分峰占总强度的分数已标在图8中。与0.05 mol/L H2SO4溶液相比,合金在0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液形成的钝化膜中Al2O3占比较高,Cr2O3含量偏低,Cr(OH)3含量上升,引起钝化膜致密性下降,这与M-S曲线测试结果一致;CoO含量下降,Co0含量上升,其他Co存在形式的占比变化不大,但一般认为Co对酸性溶液中形成的钝化膜贡献较小;Fe
3.2 AlCoCrFeNi2.1EHEA的局部腐蚀机理
图9为AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的腐蚀机理示意图。图9a1~a3为合金在0.05 mol/L H2SO4溶液中腐蚀前、亚稳态点蚀的形成和选择性腐蚀形成过程;图9b1~b3为合金在0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中腐蚀前、亚稳态点蚀的形成和稳态点蚀的形成的过程。在0.05 mol/L H2SO4溶液中,H+优先破坏B2相上的钝化膜,随着腐蚀的进一步发生,B2相钝化膜遭到均匀破坏(图9a1和a2),B2相发生均匀腐蚀(图9a3)。而在0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中,Cl-吸附在B2相上钝化膜的局部区域并对其产生破坏(图9b1),形成亚稳态点蚀(图9b2),最终诱发点蚀的形核与生长(图9b3)。依据电化学测试与腐蚀微观形貌观察,AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中钝化区变窄,Eb明显下降并有较大的点蚀倾向;局部腐蚀优先发生在富Ni-Al的B2相,而fcc相并未出现明显溶解,这主要是B2相的Al含量较高导致疏松的Al2O3在该相上形成的钝化膜中占比较大,使钝化膜的致密性及保护性能下降。AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中出现明显的点蚀,而在0.05 mol/L H2SO4溶液中并未出现这一现象,主要由于Cl-富集于点蚀坑导致坑内金属与外部金属形成浓差电偶,而且Cl-阻碍了钝化膜的再修复导致钝化膜破损区域与完整钝化膜区域形成微电偶,最终导致亚稳态点蚀发展为稳态点蚀。
图9
图9
AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的局部腐蚀机理示意图
Fig.9
Schematics of localized corrosion mechanism of AlCoCrFeNi2.1 EHEA in 0.05 mol/L H2SO4 solution (a1-a3) and 0.05 mol/L H2SO4 + 0.02 mol/L NaCl solution (b1-b3)
(a1, b1) before corrosion
(a2, b2) the form of metastable pitting
(a3, b3) selective corrosion (a3) and the form of stable pitting (b3)
4 结论
(1) AlCoCrFeNi2.1 EHEA在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中的腐蚀均包含活化-钝化-过钝化过程,少量的Cl-显著降低AlCoCrFeNi2.1 EHEA的破钝电位,轻微加速了阴极反应,但对维钝电流密度与自腐蚀电位影响不大。
(2) 在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中,AlCoCrFeNi2.1 EHEA的钝化膜均表现p-n型半导体性质,较高的极化电位导致钝化膜的缺陷浓度降低,少量的Cl-可导致钝化膜的缺陷浓度升高。
(3) 在0.05 mol/L H2SO4溶液和0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中,AlCoCrFeNi2.1 EHEA的钝化膜的主要成分为金属氧化物和氢氧化物;Cl-主要通过改变钝化膜中Cr2O3和Al2O3含量进而影响钝化膜的性质。
(4) 在0.05 mol/L H2SO4溶液中,AlCoCrFeNi2.1 EHEA的腐蚀以富Ni-Al的B2相选择性溶解为主;在0.05 mol/L H2SO4 + 0.02 mol/L NaCl溶液中,AlCoCrFeNi2.1 EHEA的腐蚀表现为点蚀与选择性溶解,2者均优先发生于富Ni-Al的B2相。
参考文献
Microstructure and mechanical properties of a FeMnCoCr high-entropy alloy with heterogeneous structure
[J].Growing attention has been placed on high-entropy alloys (HEAs) owing to their promising mechanical properties. Particularly, HEAs in which the main crystal structure is fcc are attracting significant attention. Although such alloys exhibit a good combination of strength and ductility, they cannot meet the increasing demands of applications because of limited yield strengths. In recent years, researchers have tried to improve yield strengths of HEAs by refining grains and introducing interstitial atoms. However, the processing cost is high and is often accompanied by the significant loss of ductility. In this study, we propose a simple processing route incorporating cold rolling at medium thickness reductions and short-time annealing at medium temperatures to obtain a heterogeneous structure in Fe-Mn based HEAs consisting of deformed grains with an average diameter of several tens of microns and recrystallized ultrafine grains. By simultaneously introducing multiple strengthening mechanisms, including the strengthening contributed by the microstructural characteristics of dense dislocations, grain refinement, precipitates, ε-martensite, α-martensite, and recovery twins, as well as the strengthening induced by deformation twinning and ε-martensite phase transition that occurs continuously during deformation, the yield strength of the alloy significantly increases compared with that of the fully recrystallized material and reaches 825 MPa. Simultaneously, due to the activation of significant deformation twinning and deformation-induced martensitic transformation, the uniform elongation of the alloy is about 28.6%. The proposed material fabrication method is simple, cost-effective, and can effectively improve the mechanical properties of Fe-Mn based HEAs, providing new insight into optimizing the mechanical properties of low stacking fault energy alloys of the fcc structure.
非均匀组织FeMnCoCr高熵合金的微观结构和力学性能
[J].提出了一种简单的高熵合金加工工艺,即对Fe-Mn系高熵合金采用中等形变量冷轧和中温短时退火相结合的方法,获得了由晶粒尺寸为数十微米的形变晶粒和超细尺度再结晶晶粒组成的非均匀结构。通过向合金中同时引入由高密度位错、晶粒细化、析出相、ε-马氏体、α-马氏体和回复孪晶等微观结构特征及变形过程中持续发生的形变孪生、ε-马氏体相变引起的多种强化机制,使屈服强度较充分再结晶态显著提升并达到825 MPa。同时,在塑性变形过程中由于发生了显著的形变孪生和一定的由形变诱发的奥氏体向ε-马氏体转变,合金仍具有约28.6%的均匀延伸率,合金的综合力学性能得到有效提升。该工艺为优化以fcc结构为主的低层错能合金的力学性能提供了新思路。
A promising new class of high-temperature alloys: Eutectic high-entropy alloys
[J].High-entropy alloys (HEAs) can have either high strength or high ductility, and a simultaneous achievement of both still constitutes a tough challenge. The inferior castability and compositional segregation of HEAs are also obstacles for their technological applications. To tackle these problems, here we proposed a novel strategy to design HEAs using the eutectic alloy concept, i.e. to achieve a microstructure composed of alternating soft fcc and hard bcc phases. As a manifestation of this concept, an AlCoCrFeNi2.1 (atomic portion) eutectic high-entropy alloy (EHEA) was designed. The as-cast EHEA possessed a fine lamellar fcc/B2 microstructure, and showed an unprecedented combination of high tensile ductility and high fracture strength at room temperature. The excellent mechanical properties could be kept up to 700 degrees C. This new alloy design strategy can be readily adapted to large-scale industrial production of HEAs with simultaneous high fracture strength and high ductility.
Materials science: Share corrosion data
[J].
Long-term corrosion monitoring of carbon steels and environmental correlation analysis via the random forest method
[J].In this work, the atmospheric corrosion of carbon steels was monitored at six different sites (and hence, atmospheric conditions) using Fe/Cu-type atmospheric corrosion monitoring technology over a period of 12 months. After analyzing over 3 million data points, the sensor data were interpretable as the instantaneous corrosion rate, and the atmospheric “corrosivity” for each exposure environment showed highly dynamic changes from the C1 to CX level (according to the ISO 9223 standard). A random forest model was developed to predict the corrosion rate and investigate the impacts of ten “corrosive factors” in dynamic atmospheres. The results reveal rust layer, wind speed, rainfall rate, RH, and chloride concentration, played a significant role in the corrosion process.
Mechanical properties and corrosion resistance characterization of a novel Co36Fe36Cr18Ni10 high-entropy alloy for bioimplants compared to 316L alloy
[J].
Corrosion resistance enhancement of CoCrFeMnNi high-entropy alloy fabricated by additive manufacturing
[J].
Influence of carbon on the corrosion behaviour of interstitial equiatomic CoCrFeMnNi high-entropy alloys in a chlorinated concrete solution
[J].
Corrosion of Alx CoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior
[J].
Corrosion behaviors of FeCoNiCrx (x = 0, 0.5, 1.0) multi-principal element alloys: Role of Cr-induced segregation
[J].
Evolution of microstructure, hardness, and corrosion properties of high-entropy Al0.5CoCrFeNi alloy
[J].
A new strategy to design eutectic high-entropy alloys using mixing enthalpy
[J].
A new strategy to design eutectic high-entropy alloys using simple mixture method
[J].
Grouping strategy in eutectic multi-principal-component alloy
[J].
Phase selection and mechanical properties of directionally solidified AlCoCrFeNi2.1 eutectic high-entropy alloy
[J].
Simultaneously enhanced strength-ductility of AlCoCrFeNi2.1 eutectic high-entropy alloy via additive manufacturing
[J].
Effects of the element La on the corrosion properties of CrMnFeNi high entropy alloys
[J].
Study of Al alloy corrosion in neutral NaCl by the pitting scan technique
[J].
Corrosion resistant nanostructured eutectic high entropy alloy
[J].
The effect of molybdenum on the corrosion behaviour of the high-entropy alloys Co1.5CrFeNi1.5Ti0.5Mox in aqueous environments
[J].
The corrosion behavior and film properties of Al-containing high-entropy alloys in acidic solutions
[J].
Electrochemical study of corrosion inhibition and adsorption behaviour for pure iron by polyacrylamide in H2SO4: Synergistic effect of iodide ions
[J].
Effect of the aluminium content of Alx CrFe1.5MnNi0.5 high-entropy alloys on the corrosion behaviour in aqueous environments
[J].
Electrochemical passive properties of Alx CoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids
[J].
Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution
[J].
Passivity of Sanicro28 (UNS N-08028) stainless steel in polluted phosphoric acid at different temperatures studied by electrochemical impedance spectroscopy and Mott-Schottky analysis
[J].
Inhibition effect of 2-amino-4-methylpyridine on mild steel corrosion: Experimental and theoretical investigation
[J].
Corrosion behavior and surface characterization of an equiatomic CoCrFeMoNi high-entropy alloy under various pH conditions
[J].
X-ray photoelectron spectroscopy and electrochemical investigation of the passive behavior of high-entropy FeCoCrNiMox alloys in sulfuric acid
[J].
Effect of temperature on passive film formation of UNS N08031 Cr-Ni alloy in phosphoric acid contaminated with different aggressive anions
[J].
The non-linear fitting method to analyze the measured M-S plots of bipolar passive films
[J].
Effect of roughness on general corrosion and pitting of (FeCoCrNi)0.89(WC)0.11 high-entropy alloy composite in 3.5wt.%NaCl solution
[J].
Electrochemical investigation and ab initio computation of passive film properties on copper in anaerobic sulphide solutions
[J].
Study of the surface oxides and corrosion behaviour of an equiatomic CoCrFeMnNi high entropy alloy by XPS and ToF-SIMS
[J].
Effect of annealing temperature on microstructure and corrosion behavior of CoCrFeMnNi high-entropy alloy in alkaline soil simulation solution
[J].
Microstructural characterization and corrosion behavior of Alx CoCrFeNi high entropy alloys
[J].
Galvanic corrosion in a eutectic high entropy alloy
[J].
Influence of grain refinement on the corrosion behavior of metallic materials: A review
[J].
Pitting corrosion of aluminum
[J].
Corrosion of reinforcing steel in simulated concrete pore solutions: Effect of carbonation and chloride content
[J].
Microstructure and corrosion behavior of FeCrNiCoMnx (x = 1.0, 0.6, 0.3, 0) high entropy alloys in 0.5 M H2SO4
[J].
Effect of Zr on phase separation, mechanical and corrosion behavior of heterogeneous CoCrFeNiZrx high-etropy alloy
[J].
Microstructure and corrosion behavior of (CoCrFeNi)95Nb5 high-entropy alloy coating fabricated by plasma spraying
[J].In this paper, the (CoCrFeNi)95Nb5 high-entropy alloy (HEA) coating with a thickness of 500 μm on Q235 steel substrate was fabricated by plasma spraying. The microscopic results showed that a new Laves phase is formed in the (CoCrFeNi)95Nb5 HEA coating compared to the HEA powder, and elemental segregation occurs between the dendrites and the interdendrites of the coating, while the interdendritic phase enriches with the Cr and Nb. The phase composition change and elemental segregation behavior were mainly due to the faster cooling rate of the plasma spraying technique. At the junction of the coating and the substrate, the HEA coating bonded well to the substrate; in addition, the width of transition zone was merely 2 μm. The microhardness of the (CoCrFeNi)95Nb5 HEA coating was 321 HV0.5, which is significantly higher than that of the substrate. In terms of corrosion resistance, the (CoCrFeNi)95Nb5 HEA coating has good corrosion resistance in NaCl solution. Although the corrosion form was pitting corrosion, the pitting potential of the (CoCrFeNi)95Nb5 HEA coating was significantly higher than that of other coatings, which was mainly because of the dense passivation film formed by Cr and Nb on the surface of the coating. Once the passivation film was destroyed by Cl−, the selective corrosion occurred on the surface of the (CoCrFeNi)95Nb5 HEA coating. In summary, the (CoCrFeNi)95Nb5 HEA coating prepared by plasma spraying technology can significantly improve the corrosion resistance and mechanical properties of the Q235 steel substrate.