合金高温氧化是一个复杂的过程,受材料的组织特征以及氧化环境的影响,O元素与金属基体之间产生不同的相互作用,进而导致氧化机理的改变[11~16]。影响Ni3Al基合金氧化过程的因素主要包括:合金性质[12~14]、界面及氧化环境[15,16]等。其中,合金性质(如溶质原子种类及浓度、晶粒尺寸[17,18]、微观组织[19,20]、相组成等)对合金的高温氧化行为影响显著。因此,本工作选取高Fe、Cr含量的多相Ni3Al基高温合金作为研究对象,通过合理的热处理工艺,获得3种微区结构(枝晶干γ' + γ两相组织、枝晶间β相、γ'包覆层组织),在1000℃下进行等温氧化实验,研究了Ni3Al基高温合金不同微区的高温氧化行为。
1 实验方法
实验所用多相Ni3Al基高温合金采用真空感应熔炼结合电渣重熔技术的双联冶炼工艺制备,其主要化学成分(质量分数,%)为:Fe 11.1,Al 9.2,Cr 6.7,Mo 1.3,Hf 0.3,C 0.077,B 0.018,Ti 0.009,Ni余量。制备好的铸锭经线切割制成尺寸为14 mm × 7 mm × 3 mm的试样,将试样置于电阻炉中加热至1160℃保温10 h,随炉冷却,然后进行时效处理,在800℃下保温5 h,水冷。将热处理后的试样用砂纸逐级研磨至3000号,用1.5 μm金刚石悬浮液机械抛光。氧化实验前,用丙酮超声清洗样品10 min,最后烘干。以10℃/min的升温速率将马弗炉从50℃升温至1000℃,将处理好的样品以点接触的方式放入石英坩埚中,氧化时间分别为10 min、30 h和100 h。氧化后,试样随坩埚取出,自然冷却至室温。
采用配备能谱仪(EDS)的JSM-7800F扫描电子显微镜(SEM)对氧化表面的微观结构和形貌进行表征。在配有CuKα 电极(40 kV,200 mA,波长λ = 0.15418 nm)的Smartlab-9 kW掠入射X射线衍射仪(XRD)上分析Ni3Al基高温合金的表面氧化产物,衍射角(2θ)为2°~90°,步长为0.02°。采用Renishaw inVia Raman光谱对样品表面氧化层的组成进行表征,激光波长为633 nm。采用ULVAC-PHI 700纳米扫描Auger系统对合金氧化后不同微区的氧化膜进行深度剖析,溅射深度通过相对于SiO2的仪器溅射速率(17 nm/min)估算得到。采用3D Quanta Nanolab FIB/SEM制备氧化膜的横截面透射电子显微镜(TEM)薄片,利用Tecnai G2 F30型TEM观察3个区域氧化膜截面的微观结构。
2 实验结果与讨论
2.1 热处理后的组织
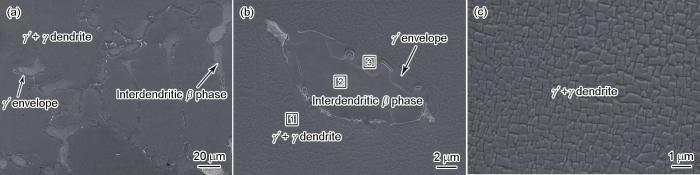
图1
图1
Ni3Al基高温合金热处理后显微组织的SEM像
Fig.1
SEM image of the Ni3Al-based superalloy after heat treatment, showing γ' + γ dendrite, interdendritic β phase, and γ' envelope (a); a higher magnification SEM image of interdendritic β phase and γ' envelope (b); a higher magnification SEM image of γ' + γ dendrite, showing cubic γ' precipitates separated by γ channels (c)
表1 Ni3Al基高温合金不同区域化学成分的EDS分析 (atomic fraction / %)
Table 1
| Position in Fig.1b | Al | Fe | Cr | Ni |
|---|---|---|---|---|
| 1 (γ' + γ dendrite) | 8.5 | 18.6 | 12.4 | 60.5 |
| 2 (interdendritic β phase) | 27.2 | 8.9 | 1.5 | 62.4 |
| 3 (γ' envelope) | 19.4 | 6.6 | 2.2 | 71.8 |
2.2 氧化后的表面形貌
图2
图2
Ni3Al基高温合金在1000℃氧化10 min后表面形貌的SEM像
Fig.2
Surface SEM image of the Ni3Al-based superalloy oxidized at 1000oC for 10 min (a) and higher magnification surface SEM images of interdendritic β phase and γ' envelope (b), interdendritic β phase (c), and γ' + γ dendrite (d)
图3
图3
Ni3Al基高温合金在1000℃氧化30及100 h后表面形貌的SEM像
Fig.3
Low (a, c) and high (b, d) magnified surface SEM images of the Ni3Al-based superalloy oxidized at 1000oC for 30 h (a, b) and 100 h (c, d)
2.3 氧化膜的结构及组成
Ni3Al基高温合金在1000℃氧化10 min和100 h的XRD谱如图4所示。在氧化初期(10 min),氧化产物主要为NiO、α-Al2O3和θ-Al2O3 (下文简称Al2O3)以及NiFe2O4相。当氧化时间延长到100 h后,氧化膜主要组成则为α-Al2O3和θ-Al2O3。
图4
图4
Ni3Al基高温合金在1000℃氧化10 min和100 h的XRD谱
Fig.4
XRD spectra of the Ni3Al-based superalloy oxidized at 1000oC for 10 min and 100 h
图5
图5
Ni3Al基高温合金在1000℃氧化10 min和100 h后不同区域表面氧化膜的Raman光谱
Fig.5
Raman spectra of surface oxide scales in different regions of the Ni3Al-based superalloy oxidized at 1000oC for 10 min (a) and 100 h (b)
图6
图6
Ni3Al基高温合金在1000℃氧化10 min后不同微区的Auger电子能谱(AES)元素深度分布
Fig.6
Auger electron spectrum (AES) element-depth profiles of Ni3Al-based superalloy oxidized at 1000oC for 10 min
(a) γ' + γ dendrite
(b) interdendritic β phase
(c) γ' envelope
2.4 不同微区截面氧化膜的表征
枝晶干γ' + γ两相区在1000℃氧化10 min后截面形貌的TEM像、选区电子衍射(SAED)花样和EDS元素面扫图如图7所示。结合枝晶干γ' + γ两相区相关Raman光谱和AES分析,可知此处仅形成了少量的NiO和NiFe2O4,主要为Al2O3,可近似认为氧化膜为单一的Al2O3层。
图7
图7
枝晶干γ' + γ在1000℃氧化10 min后截面形貌的TEM像、选区电子衍射(SAED)花样以及框线区域的EDS元素面扫分布
Fig.7
Cross-sectional TEM image of γ' + γ dendrite oxidized at 1000oC for 10 min (a) and the EDS element mapping of the frame area depicting the distributions of elements O (b), Al (c), Cr (d), Fe (e), and Ni (f) (Inset in Fig.7a shows the selected area electron diffraction (SAED) pattern of γ' + γ dendrite)
图8为枝晶间β相在1000℃氧化10 min后截面形貌的TEM像、SAED花样和EDS元素面扫图。此微区形成了单一的Al2O3层,外部没有Fe、Ni元素的富集,相比于枝晶干γ' + γ两相区,此处的Al2O3层更为平直,厚度略薄。
图8
图8
枝晶间β相在1000℃氧化10 min后截面形貌的TEM像、SAED花样以及框线区域的EDS元素面扫分布
Fig.8
Cross-sectional TEM image of interdendritic β phase oxidized at 1000oC for 10 min (a) and the EDS element mapping of the frame area depicting the distributions of elements O (b), Al (c), Cr (d), Fe (e), and Ni (f) (Inset in Fig.8a shows the SAED pattern of interdendritic β phase)
图9
图9
γ'包覆层在1000℃氧化10 min后截面形貌的TEM像、SAED花样以及相应的EDS元素面扫分布
Fig.9
Cross-sectional TEM image of γ' envelope oxidized at 1000oC for 10 min (a) and the corresponding EDS element mapping depicting the distributions of elements O (b), Al (c), Cr (d), Fe (e), and Ni (f) (Inset in Fig.9a shows the SAED pattern of γ' envelope, and the rectangular frames in Fig.9a show the holes)
当氧化时间达到30 h以后时,3个微区则呈现出相对均匀一致的氧化膜特征,如图10所示。氧化膜为单一的Al2O3,厚度约为1.5 μm。
图10
图10
Ni3Al基高温合金在1000℃氧化30 h截面形貌的SEM-BSE像和相应的EDS元素分布
Fig.10
Cross-sectional SEM-BSE image of Ni3Al-based superalloy oxidized at 1000oC for 30 h (a) and the corresponding EDS element mapping depicting the distributions of elements O (b), Al (c), and Ni (d)
图11
图11
Ni3Al基高温合金在1000℃氧化100 h截面形貌的SEM-BSE像和相应的EDS元素分布
Fig.11
Cross-sectional SEM-BSE image of Ni3Al-based superalloy oxidized at 1000oC for 100 h (a) and the corresponding EDS element mapping depicting the distributions of elements O (b), Al (c), and Ni (d)
2.5 不同微区的氧化行为
通过以上分析结果可知,在1000℃等温氧化过程中,合金氧化初期(10 min) 3个微区呈现出不同的氧化行为。其中γ'包覆层氧化程度最为严重,为明显的双层结构,外部由混合的NiO、Al2O3和NiFe2O4组成,内部是单一的Al2O3层;枝晶干γ' + γ两相区和枝晶间β相则可近似看成单一的Al2O3。高温合金中γ'相是有序的L12结构的fcc相,晶格常数约为0.359 nm[29]。枝晶间β相是有序B2结构的bcc相,晶格常数约为0.289 nm[30],2者具有不同的晶体结构,枝晶间β相与γ'包覆层之间的相界成为金属离子和O2-的快速扩散通道[31~33]。在氧化初期,Ni2+、Fe3+在γ'包覆层和枝晶间β相界面处快速扩散,界面处的快速扩散通道导致γ'包覆层优先氧化,NiO和NiFe2O4的保护性较差,呈现明显的胞状凸起形貌特征。
随着氧化时间的延长,γ'包覆层的胞状氧化物并没有持续长大,而是逐渐变为细小的颗粒状,3个微区的氧化膜逐渐趋于一致,氧化膜由致密的颗粒状Al2O3组成。同时其厚度随氧化反应的进行而增加,由氧化30 h的1.5 μm生长到氧化100 h的2.3 μm。这是因为Al3+的扩散速率对于温度更为敏感,当氧化温度较高时,晶格扩散取代界面/晶界扩散成为控制氧化速率的主要因素,高温促进了Al3+通过晶格向外扩散。此外,在高温下各元素除了发生氧化反应生成NiO、NiFe2O4和Al2O3等氧化物外,还会发生还原夺氧反应。由热力学可知,生成Gibbs自由能(ΔG)愈负,该金属的氧化物愈加稳定,金属还原夺氧能力愈强(氧活性愈高)。以NiO和Al2O3为例,生成氧化物的反应如下:
高温下,Al持续不断地向外部氧化层扩散,这就使得NiO不断地被还原,Al2O3含量不断增加,而NiO逐渐减少,同时Al元素还会直接与渗入的氧发生反应生成Al2O3。因此,γ'包覆层处氧化膜中Al2O3的含量逐渐增加,而NiO、NiFe2O4的生成量减少,导致表面氧化膜不再呈现显著的胞状凸起。最终随着氧化时间的增加,3个微区氧化行为趋于一致。
3 结论
多相Ni3Al基高温合金中主要有3种不同的微区结构:枝晶干γ' + γ两相区、枝晶间β相和包裹着枝晶间β相的γ'包覆层。1000℃等温氧化初期(氧化10 min),3个微区呈现出不同的氧化行为:γ'包覆层处优先氧化,氧化膜为明显的双层结构,为胞状凸起形貌,外层主要由NiO、NiFe2O4和Al2O3组成,内层为单一的Al2O3层;而枝晶干γ' + γ两相区和枝晶间β相则为单层Al2O3。随着等温氧化时间的延长(氧化30和100 h),由于Al的还原夺氧反应,γ'包覆层氧化初期形成的胞状氧化物转变为相对均匀致密的Al2O3,3个微区的氧化形貌逐渐趋于一致。3个微区表面Al2O3膜厚度随着氧化反应的进行而缓慢生长,由氧化30 h时的1.5 μm增加到氧化100 h的2.3 μm。
参考文献
Advances in processing of Ni3Al-based intermetallics and applications
[J].
The effect of long term high temperature annealing on twinning and detwinning of the wrought Ni3Al-based alloy
[J].
High-pressure strengths of Ni3Al and Ni-Al-Cr
[J].
Ultrahigh-temperature materials for jet engines
[J].This introductory article provides the background for the September 2003 issue ofMRS Bulletinon Ultrahigh-Temperature Materials for Jet Engines. It covers the need for these materials, the history of their development, and current challenges driving continued research and development. The individual articles that follow review achievements in four different material classes (threein situcomposites—based on molybdenum silicide, niobium silicide, and silicon carbide, respectively—and high-melting-point platinum-group-metal alloys), as well as advances in coating systems developed both for oxidation protection and as thermal barriers. The articles serve as a benchmark to illustrate the progress made to date and the challenges ahead for ultrahigh-temperature jet-engine materials.
Precipitate coarsening and its effects on the hot deformation behavior of the recently developed γ'- strengthened superalloys
[J].
Effects of heat treatment on the microstructure and mechanical properties of Ni3Al-based superalloys: A review
[J].
Recent progress of microstructure evolution and performance of multiphase Ni3Al-based intermetallic alloy with high Fe and Cr contents
[J].
高Fe、Cr含量多相Ni3Al基高温合金组织与性能研究进展
[J].
Microstructure-dependent oxidation behavior of Ni-Al single-crystal alloys
[J].The effect of the γ′+γ two-phase structure on the oxidation behaviors of Ni-Al single-crystal alloys at 650 °C was investigated by scanning electron microscopy, transmission electron microscopy, atomic force microscopy, X-ray diffraction and Auger electron spectroscopy. In the initial oxidation stage, the oxidation behavior is primarily determined by the growth pattern of oxides in the γ channel. The outward convex NiO was formed in unprotected wide γ channels. And Ni-Al spinel oxide provides a great number of short-circuit paths, accelerating the inward diffusion of oxygen and outward diffusion of Ni. In the late stage of oxidation, the elongated internal oxide in the large γ′ phase contributes to the diffusion of oxygen along the oxide/metal interface. Consequently, the Ni-Al single-crystal alloy with wide γ channels and large γ′ precipitates exhibited poor oxidation performance.
Surface oxidation and ductility loss in boron-doped Ni3Al at 760oC
[J].
Dynamic embrittlement of boron-doped Ni3Al alloys at 600oC
[J].
High-temperature oxidation behavior of modified 4Al alumina-forming austenitic steel: Effect of cold rolling
[J].The oxidation behavior and mechanism of as-received and 30 % cold-rolled alumina-forming austenitic (AFA) steel were investigated in dry air at 700 °C. The results show that the mass gain per unit area curves of as-received and 30 % cold-rolled steels subject to near-parabolic law before 100 h oxidation time. Two samples both show higher high-temperature oxidation resistance due to the formation of dense Al2O3 oxide scale. Gradual spallation of outer scale results in the formation of continuous and dense alumina scale. Dislocations can act as short-circuit diffusion channel for the diffusion of Al from alloy matrix to surface, and also provide nucleation sites for B2-NiAl phase, which ensure the continuous formation of Al2O3 scale.
Compositional factors affecting the oxidation behavior of Pt-modified γ-Ni plus γ'-Ni3Al-based alloys and coatings
[J].
High-temperature oxidation behavior of pure Ni3Al
[J].
High temperature oxidation behaviours of three zirconium alloys
[J].
3种锆合金的高温氧化行为
[J].
High-temperature oxidation of reactively processed nickel aluminide intermetallics
[J].
Initial oxidation behavior of Ni3Al (210) surface induced by supersonic oxygen molecular beam at room temperature
[J].
Rietveld refinement, microstructure and high-temperature oxidation characteristics of low-density high manganese steels
[J].Three forged low-density high manganese steels Mn28Al10, Mn28Al8 and Mn20Al10 were used as experimental materials in this study. The forged microstructure and external oxidation characteristics at 1323 K and 1373 K for 5-25 h in air were investigated by microstructural observation and X-ray diffraction (XRD) technique. The phase compositions and abundance in the forged and oxidized samples were quantitatively obtained by Rietveld method on the basis of XRD pattern analysis. The results showed that an austenitic microstructure formed in steels Mn28Al10 and Mn28Al8, and 18.02 wt% ferrite could be found in Mn20Al10. The relative amount of ~5.28 wt% κ-carbide (Fe3AlC0.5) in Mn28Al10 was far greater than that in Mn28Al8 and Mn20Al10. The oxidation kinetics of forged steels oxidized at 1323 K for 5-25 h had two-stage parabolic rate laws; and the oxidation rate of the first stage was lower than that of the second stage. When they were oxidized at 1373 K for 5-25 h, the oxidation kinetics followed only a parabolic law and the oxidation rates were respectively greater than those at 1323 K for 5-25 h. When they were oxidized at 1323 K for 25 h, detached external scales contained Fe2MnO4 and α-Fe2O3 oxides. α-Al2O3 and (Fe, Mn)2O3 oxides could only be indexed in steels Mn28Al8 and Mn28Al10, respectively. When they were oxidized at 1373 K for 25 h, Fe2MnO4, Fe3O4, α-Fe2O3 and α-Al2O3 oxides could all be indexed in the external detached scales. The main phase of detached external scales was Fe2MnO4; and the relative amount of α-Al2O3 in steel Mn28Al8 was higher than that in steels Mn28Al10 and Mn20Al. The external oxidation layers of these three forged steels oxidized at 1323 K and 1373 K for 25 h were essentially followed the sequence of α-Al2O3, Fe2MnO4, Fe3O4, FeMnO3, and Fe2O3 from the substrate to the outside surface. The forged Mn28Al10 steel with austenitic microstructure and a certain amount of κ-carbide (~5.28 wt% in the present work) possessed a better combination of strength, ductility, specific strength, and oxidation rate when compared to that of the forged Mn28Al8 and Mn20Al10 steels.
Effects of grain sizes on the oxidation behavior of Ni-based alloy 230 and N
[J].
Effect of cold rolling on the oxidation resistance of T91 steel in oxygen-saturated stagnant liquid lead-bismuth eutectic at 450oC and 550oC
[J].
Effect of microstructure on the oxidation behavior of two nickel-based superalloys
[J].
Effect of water drops on the oxidation mechanisms of a ceria coated nickel-based superalloy
[J].
Composition, structure and properties of the oxide films formed on the stainless steel 316L in a primary type PWR environment
[J].
In situ Raman spectroscopic investigation of stainless steel hydrothermal corrosion
[J].
Isothermal oxidation behavior of micro-regions in multiphase Ni3Al-based superalloys
[J].
In situ Raman spectroscopic investigation of nickel hydrothermal corrosion
[J].
Development of an in situ Raman spectroscopic system for surface oxide films on metals and alloys in high temperature water
[J].
Microstructure analysis of initial alumina of Pt-modified aluminide coatings on Ni-based alloy
[J].
镍基合金表面Pt改性铝化物涂层的初期Al2O3微观结构分析
[J].
Characterization of pore development at Al2O3/FeAl interface during high temperature oxidation
[J].
高温氧化时Al2O3/FeAl界面孔洞形成过程的分析
[J].
Microstructures and mechanical properties of a directionally solidified NiAl-Mo eutectic alloy
[J].
Determination of oxygen diffusion along nickel/zirconia phase boundaries by internal oxidation
[J].
Oxidation behavior of Ni-Cr-Fe-based alloys: Effect of alloy microstructure and silicon content
[J].
In-situ environmental TEM study of γ'-γ phase transformation induced by oxidation in a nickelbased single crystal superalloy
[J].