目前已发展的高温形状记忆合金,如Ti-Ni-X (X = Pd、Pt、Au、Hf、Zr)、Ti-Ta、Ni-Mn-Ga、TaRu和NbRu等[8~13],均因成本较高而无法大规模应用。Cu-Al-Ni高温形状记忆合金成本低廉,具有优良的单晶形状记忆效应和超弹性,成为高温形状记忆合金实用化的优秀候选材料[14,15]。Cu-Al-Ni合金最大的应用障碍,在于该合金的晶粒粗大,造成晶界偏析等缺陷,使晶界脆化并易发生沿晶断裂,表现出严重的多晶脆性[16~21]。研究者[15,22,23]主要通过粉末冶金、定向凝固或者合金化的方法来改善多晶Cu-Al-Ni合金的力学性能,其中添加第四组元合金化的方法最为简单有效。
1 实验方法
实验用合金的名义成分为Cu-13Al-4Ni-xY (x = 0.2、0.5,质量分数,%)。选择纯度为99.99%的4种原材料按比例配好,使用非自耗真空电弧炉进行熔炼,为保证材料熔炼均匀,熔炼过程中翻转材料8次,得到钮扣状铸锭,然后进行真空封管,在850℃均匀化处理12 h,冰水淬火。
热处理后将材料切割出8 mm × 8 mm × 2 mm的片状样品,利用D/max-rB X射线衍射仪(XRD)进行分析,扫描速率为4°/min,范围为30°~80°。相同样品经打磨、抛光、腐蚀后,利用Axioscope A1金相显微镜(OM)和MERLIN Compact场发射扫描电子显微镜(SEM)进行显微组织观察。OM和SEM样品使用的腐蚀液为100 mL蒸馏水 + 30 mL HCl + 10 g FeCl3。利用配有能谱 (EDS)分析附件的Tecnai G2 F20场发射透射电子显微镜(TEM)进行成分与结构分析,样品在2.5 g FeCl3·6H2O + 10 mL HCl + 48 mL CH3OH组成的溶液中进行电解双喷减薄,减薄温度-25℃。利用EXSTAR6000 TG/DTA6300热重差热综合热分析仪(DTA)测量马氏体相变温度,升温和降温速率均为10℃/min。结果表明,Cu-13Al-4Ni-0.2Y合金和Cu-13Al-4Ni-0.5Y合金的马氏体开始转变温度(Ms)分别为135和174℃。
利用压缩法在GNT50电子万能试验机上测试试样的力学性能和形状记忆效应,样品为直径3 mm、长5 mm的圆棒,横梁移动速率为0.2 mm/min。形状记忆效应的计算方法为:材料原长L0,预加载后长度压缩至L1,卸载后由于回弹,长度回复到L2,之后加热到马氏体逆相变温度之上,发生形状回复,长度为L3;则预应变ε = (L0 - L1) / L0 × 100%,形状记忆效应SME = (L3– L2) / L0 × 100%。
利用Autolab电化学工作站进行电化学分析。电化学测量采用三电极体系,样品为工作电极,Pt片为对电极,Ag/AgCl电极为参比电极(在25℃下,相对于氢标准电极的电极电位为+ 0.210 V),测试溶液为3.5%NaCl溶液。对用2000号砂纸打磨过的样品进行5 min的阴极除膜过程,然后进行30 min的开路电位(OCP)测试,最后进行动电位极化测试,测量范围为相对于开路电位± 0.5 V (vs Ag/AgCl),步阶为1 mV,扫描速率为0.5 mV/s。
2 实验结果与分析
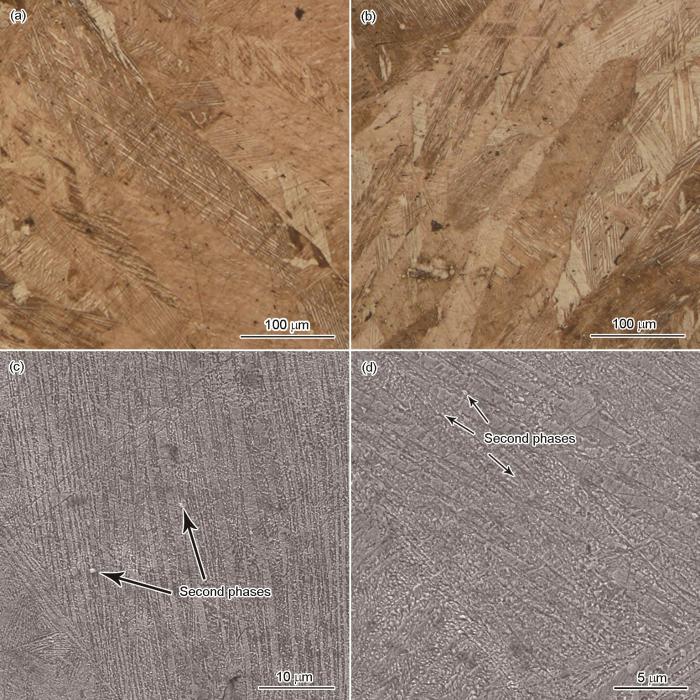
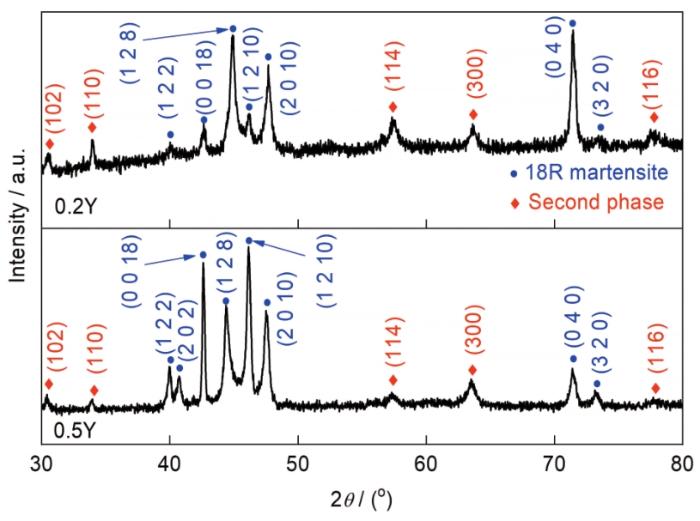
图1分别给出了Cu-13Al-4Ni-xY (x = 0.2、0.5)合金的OM和SEM像。如图1a和b所示,Cu-13Al-4Ni-xY合金的晶粒尺寸为100~200 μm,远小于Cu-13Al-4Ni合金的晶粒尺寸(1~3 mm)[24]。已知室温下Cu-13Al-4Ni合金的组织包含板条状2H马氏体和人字形18R马氏体[24],通过XRD结果(图2)可以看出,Y元素添加后,Cu-13Al-4Ni-xY合金的室温组织由18R马氏体和类似六方结构Cu4Y的第二相组成。从SEM像(图1c和d)可见,Cu-13Al-4Ni-xY合金的马氏体组织属于典型的18R马氏体,第二相分散在18R马氏体中,而2H马氏体消失。
图1
图1
Cu-13Al-4Ni-xY (x = 0.2、0.5)合金的OM和SEM像
Fig.1
OM (a, b) and SEM (c, d) images of Cu-13Al-4Ni-xY alloys with x = 0.2 (a, c) and x = 0.5 (b, d)
图2
图2
Cu-13Al-4Ni-xY (x = 0.2、0.5)合金的XRD谱
Fig.2
XRD spectra of Cu-13Al-4Ni-xY (x = 0.2 and 0.5) alloys
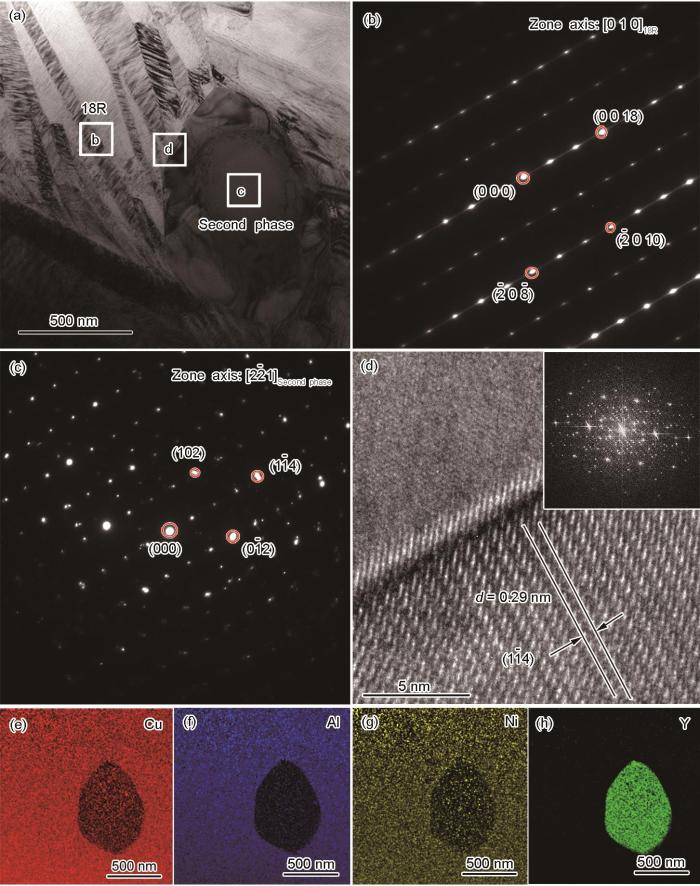
XRD结果表明,Cu-13Al-4Ni-0.2Y合金与Cu-13Al-4Ni-0.5Y合金的相组成基本一致,且Cu-13Al-4Ni-0.5Y合金中的第二相颗粒较多(如图1中箭头所示),因此对Cu-13Al-4Ni-0.5Y合金进行TEM观察,以进一步确定Cu-13Al-4Ni-xY合金的相组成,结果如图3所示。由图3a~c可知,类似六方Cu4Y的第二相粒子嵌在18R马氏体中,尺寸约为500 nm。高分辨图像(图3d)显示,在(1
图3
图3
Cu-13Al-4Ni-0.5Y合金的TEM明场像、SAED花样、HRTEM像及第二相区域EDS元素面扫描图
Fig.3
TEM bright field image (a), the corresponding selected area electron diffraction (SAED) patterns of 18R martensite (b) and second phase (c) in the Cu-13Al-4Ni-0.5Y alloy; high resolution transmission electron microscope (HRTEM) image of area d in Fig.3a (Inset shows the fast Fourier transform) (d); EDS element maps of Cu (e), Al (f), Ni (g), and Y (h) for the second phase (d—interplanar spacing)
图4a为Cu-13Al-4Ni-xY (x = 0.2、0.5)合金的压缩应力-应变曲线。可见,当x = 0.2时,压缩断裂强度为978 MPa,应变为15.6%;当x = 0.5时,压缩断裂强度和应变分别提高到1185 MPa和19.3%,远高于Cu-13Al-4Ni合金(580 MPa和10.5%)[24]。在多晶Cu-Al-Ni合金发生形变时,合金中的晶粒位向发生变化,为了保证界面上的应变连续性,在晶界处会发生应力集中,因此Cu-13Al-4Ni合金的断裂形式是沿晶断裂[21]。由于晶粒细化,Y元素大大提高了合金的强度和塑性。此外,第二相在基体中随机分布,导致位错和应力在第二相附近积累,所以Cu-13Al-4Ni-xY合金的断裂形式由未掺杂合金的沿晶断裂变为穿晶断裂,如图4b和c所示。基体中脆性2H马氏体的消失也是力学性能提高的另一个重要原因[26]。
图4
图4
Cu-13Al-4Ni-xY (x = 0.2、0.5)合金的压缩应力-应变曲线及断口形貌
Fig.4
Compressive stress-strain curves (a), and fracture morphologies of the Cu-13Al-4Ni-xY alloys with x = 0.2 (b) and x = 0.5 (c)
图5为Cu-13Al-4Ni-xY (x = 0.2、0.5)合金的应变恢复特性曲线。当预应变为8%时,x = 0.2和0.5合金的形状记忆效应分别为4.8%和4.2%,远高于Cu-13Al-4Ni合金的2.6%[24]。当预应变增加到10%时,x = 0.2合金的形状记忆效应为5.5%,x = 0.5合金的形状记忆效应为5.1%。Y掺杂提高了Cu-13Al-4Ni-xY合金的力学性能,增强了其抵抗不可逆变形的能力。因此,Cu-13Al-4Ni-xY合金的形状记忆效应得到了相应的改善。Cu-13Al-4Ni-0.5Y合金的可逆应变低于Cu-13Al-4Ni-0.2Y合金的原因在于,随着Y含量的增加,合金基体中的(Cu, Al, Ni)4Y相含量随之增多,损害了合金的形状记忆效应。
图5
图5
Cu-13Al-4Ni-xY (x = 0.2、0.5)合金分别在8%和10%预应变下的恢复特性曲线
Fig.5
Recovery characteristic curves of the Cu-13Al-4Ni-xY alloys with x = 0.2 (a) and x = 0.5 (b) under pre-strains of 8% and 10%, respectively (The arrow lines represent the recovery strain after heating to 350oC for 1 min. SME—shape memory effect)
图6
图6
Cu-13Al-4Ni-xY (x = 0、0.2、0.5)合金在3.5%NaCl溶液中的动电位极化曲线
Fig.6
Potential polarization curves for the Cu-13Al-4Ni-xY (x = 0, 0.2, 0.5) alloys in the 3.5%NaCl solution
表1 Cu-13Al-4Ni-xY合金在3.5%NaCl溶液中的腐蚀参数
Table 1
| Alloy | Ecorr (vs Ag/AgCl) | icorr | Rp | vcorr |
|---|---|---|---|---|
| V | μA·cm-2 | kΩ·cm2 | mm·a-1 | |
| Cu-13Al-4Ni[25] | -0.264 | 1.47 | 2.29 | 0.017 |
| Cu-13Al-4Ni-0.2Y | -0.271 | 2.25 | 1.40 | 0.026 |
| Cu-13Al-4Ni-0.5Y | -0.272 | 3.03 | 1.56 | 0.035 |
式中,a、b、c分别为Cu2+、Al3+、Ni2+的系数。
阳极支路表示Cu的溶解。首先Cu溶解为Cu+,然后Cu+被氧化为Cu2+[28]:
Cu-Al-Ni合金的总溶解反应表达式为:
3 结论
(1) Y元素掺杂后,Cu-13Al-4Ni合金从由2H和18R马氏体组成变为单一的18R马氏体,并且其中伴随着晶粒细化以及六方(Cu, Al, Ni)4Y相的出现。
(2) 合金的力学性能大幅提升,Cu-13Al-4Ni-0.5Y合金的压缩断裂应变和压缩断裂应力分别达到了19.3%和1185 MPa。断裂形式也从沿晶断裂变为穿晶断裂。力学性能的提高促进了形状记忆效应的改善,Cu-13Al-4Ni-0.2Y合金在预应变10%加热后,可得到5.5%的可逆应变。Cu-13Al-4Ni-xY合金的耐蚀性相较于Cu-13Al-4Ni合金稍有下降。
参考文献
Research progress in high temperature shape memory alloys
[J].
高温形状记忆合金的研究进展
[J].
Research Progress of iron-based shape memory alloys: A review
[J].
Fe基形状记忆合金的研究进展
[J].
Characterization of laser beam offset welding TiNi alloy and 304 stainless steel with different joining modes
[J].
A cyclic integrated microstructural-mechanical model for a shape memory alloy
[J].
NiTiHf-based shape memory alloys
[J].
High-temperature shape memory alloys
[J].
High temperature shape memory alloys problems and prospects
[J].
The machining characteristics and shape recovery ability of Ti-Ni-X (X = Zr, Cr) ternary shape memory alloys using the wire electro-discharge machining
[J].
Laser alloying as an effective way to fabricate NiTiPt shape memory alloys
[J].
Microstructure and martensitic transformation of Ti49Ni51 - x Hf x high temperature shape memory alloys
[J].
Deformation mechanism of Ni54Mn25Ga20.9Gd0.1 high-temperature shape memory alloy
[J].
Structural, electronic and elastic properties of the shape memory alloy NbRu: First-principle investigations
[J].
The shape memory effect in equiatomic TaRu and NbRu alloys
[J].
Thermal and pseudoelastic cycling in Cu-14.1Al-4.2Ni (wt%) single crystals
[J].
Preparation of single crystal CuAlNiBe SMA and its performances
[J].
Effects of Mn additions on the structure, mechanical properties, and corrosion behavior of Cu-Al-Ni shape memory alloys
[J].
Influences of 2.5wt% Mn addition on the microstructure and mechanical properties of Cu-Al-Ni shape memory alloys
[J].
High strength and high electrical conductivity CuMg alloy prepared by cryorolling
[J].
Effects of annealing temperature on thermomechanical properties of Cu-Al-Ni shape memory alloys
[J].
Effect of chromium element on transformation, mechanical and corrosion behavior of thermomechanically induced Cu-Al-Ni shape-memory alloys
[J].
The enhancement of the mechanical properties and the shape memory effect for the Cu-13.0Al-4.0Ni alloy by boron addition
[J].
Microstructure and mechanical properties of Cu-12Al-6Ni with Ti addition prepared by powder metallurgy
[J].
Effect of Co additions on the damping properties of Cu-Al-Ni shape memory alloys
[J].
Effects of Gd addition on the microstructure, mechanical properties and shape memory effect of polycrystalline Cu-Al-Ni shape memory alloy
[J].
Effect of Nd addition on the microstructure, mechanical properties, shape memory effect and corrosion behaviour of Cu-Al-Ni high-temperature shape memory alloys
[J].
Formation of stress-induced martensite in the presence of γ-phase, in a Cu-Al-Ni-Mn-Fe shape memory alloy
[J].
Effect of nickel content on the electrochemical behavior of Cu-Al-Ni alloys in chloride free neutral solutions
[J].
Grain refinement of a Cu-Al-Ni shape memory alloy by Ti and Zr additions
[J].