近年来,可降解金属材料(主要为镁基、铁基和锌基金属材料)由于可降解特性以及良好的力学性能,在生物医用植入物领域受到研究者的广泛关注。然而,镁基和铁基金属材料在生理环境中分别过快和过慢的腐蚀速率难以与患处愈合过程相匹配,极大限制了其在临床上的进一步应用。Zn的标准电极电位(-0.762 V vs SHE (标准氢电极))介于Mg (-2.34 V vs SHE)和Fe (-0.44 V vs SHE)之间,在理论上具有更加适中的腐蚀速率[1]。同时,体内实验结果[2]表明,Zn丝在植入鼠腹动脉4个月内保持了良好的力学完整性,随后腐蚀速率迅速增加,其腐蚀速率曲线与大部分植入物的临床要求相匹配。此外,Zn是人体内第二丰富的微量元素,在正常成年人体内Zn含量为2~3 g,而且人体每日Zn摄入量为4~14 mg,这使得锌基植入体在降解过程中释放的Zn2+安全浓度窗口大,不易产生系统毒性[3,4]。同样,Zn也是人体内所有六类酶以及部分蛋白的辅助因子,且在人体的生长发育、免疫系统、代谢和神经系统中起着重要作用[5,6]。更重要的是,Zn可以修复和提升血管内皮的完整性和抗动脉粥样硬化,并且还能够刺激成骨细胞的增殖和矿化[6~9],展现了锌基金属材料潜在的生物功能性。基于此,Zn被认为是一种极具潜力的新兴可降解金属材料。
然而,纯Zn的综合力学性能不足极大限制了其在临床上的应用。铸态纯Zn的极限抗拉强度(UTS)约为20 MPa,经塑性变形处理后可提升至120 MPa左右[10,11],但仍远不能达到植入物的临床使用要求。目前已有大量工作通过合金化与机械加工的方式尝试改善锌基金属材料力学性能不足这一问题,但大部分合金体系无法兼顾强度与韧性,与实际临床应用的性能要求仍有距离。此外,Zn在生理环境下存在腐蚀不均匀的情况,而部分合金元素的加入会加剧这一问题[12~14]。不均匀腐蚀往往会导致植入物的过早断裂,进而丧失应有的力学支撑功能。因此,亟需开发可兼顾优良综合力学性能和良好降解行为的锌基合金体系,以推动可降解锌基材料在人体植入物中的应用。
Cu是维持人体器官和代谢过程稳定的必需元素,能够刺激内皮细胞增殖和促进血管生成,并且还能促进骨骼生长[15,16]。此外,添加Cu元素可以同时增强Zn基体的强度和韧性[15],这使得Cu成为一种理想的用于提高Zn综合力学性能的合金元素。但过多的Cu添加存在生物安全问题,有报道称Zn-4Cu (质量分数,%,下同)合金在体外生物相容性实验中对成纤维细胞表现出一定的细胞毒性[17];而Zn-2Cu合金表现出良好的生物相容性[18]。此外,Cu元素含量的增加会加剧Zn基体的不均匀腐蚀[19]。Zr是一种具有良好生物相容性的元素,并有利于Zn的晶粒细化[20]。同时,热挤压工艺可有效细化锌基合金的第二相和基体相晶粒尺寸[21,22]。基于此,本工作制备了新型Zn-2Cu-0.5Zr合金,一方面既发挥主加Cu元素合金化的强化优势,又避免了其过量添加可能造成的不利影响(如细胞毒性);另一方面通过辅加Zr元素进一步强化和细化晶粒;最后,通过热挤压工艺,进一步改善合金的组织。通过以上合金化与机械热加工,实现锌基合金综合力学性能和降解行为的改善,并深入探讨了该新型合金材料“工艺-结构-性能”的关系与机制。
1 实验方法
1.1 样品制备
Zn-2Cu和Zn-2Cu-0.5Zr合金分别由高纯Zn锭(99.99%)、Cu丝(99.9%)和Zr粒(99.95%)制备而成。在680℃电阻炉中使原料熔化,将合金熔液浇注进预热至200℃的铸铁模具中,空冷至室温,得到直径85 mm、长400 mm的铸锭。将铸锭加热至350℃,保温30 min,按照22∶1的挤压比,0.4 mm/s的挤压速率对铸锭进行热挤压,挤压后空冷至室温。采用同样工艺制备热挤压纯Zn作为对照。从样品中部垂直于挤压方向切取直径10 mm、厚1.8 mm的圆片用于微观组织和降解行为表征。拉伸测试样品为沿挤压方向切取的狗骨状试样。所有样品均依次用600、1200和2000号的砂纸进行打磨,然后用无水乙醇进行超声清洗(每次5 min,共清洗3次),最后用洗耳球将表面酒精吹干,放在真空干燥箱中待用。
1.2 微观组织表征
使用粒度为1 μm的金刚石抛光膏对样品进行抛光处理,用1 g H2C2O4、1 mL HNO3、1 mL CH3COOH和150 mL去离子水配制而成的侵蚀剂对样品表面进行侵蚀,通过AxioCam ERc5s光学显微镜(OM)和JSM 7800F场发射扫描电子显微镜(SEM)对样品的微观组织进行观察。利用X'Pert Philips型X射线衍射仪(XRD)对样品相组成进行分析,XRD测试采用CuKα 射线源,衍射角2θ范围为30°~90°,步长0.033°。
1.3 力学拉伸测试
拉伸实验按照GB/T 228.1-2010《金属材料拉伸试验第1部分:室温试验方法》进行,利用WDW-3100电子万能试验机表征样品的室温力学性能,试样拉伸速率为1 mm/min,每组测试3个样品。屈服强度和极限抗拉强度由应力-应变曲线获得,断后伸长率通过测量拉伸实验前后样品的标距长度计算。
1.4 电化学腐蚀表征
在(37 ± 0.5)℃的条件下,通过IM6电化学工作站对样品进行电化学测试。电化学工作站是由饱和甘汞参比电极(SCE)、Pt对电极(1 cm × 1 cm)和工作电极(待测试样)组成的三电极系统。所有样品背面都经过仔细打磨,去除氧化物后与铜导线相连。然后用硅橡胶进行封样,将样品表面暴露在外面,暴露面积为0.79 cm2。为了模拟人体生理环境,选择Hank's溶液作为电化学测试的电解液。Hank's溶液由8.00 g NaCl、0.40 g KCl、0.14 g CaCl2、0.35 g NaHCO3、1.00 g C6H12O6、0.10 g MgCl2·6H2O、0.06 g MgSO4·7H2O、0.06 g Na2HPO4·12H2O、0.06 g KH2PO4和1 L去离子水配制而成[23],并用稀HCl将pH值调至7.4。电化学测试前,利用水浴锅将Hank's溶液升温至(37 ± 0.5)℃,将样品置于溶液中稳定0.5 h,测试过程保持温度恒定。以1 mV/s的扫描速率记录动电位极化曲线,利用Tafel外推法对动电位极化曲线进行分析(外推点选取阴极活化区电位约60 mV处),计算得出样品的腐蚀电流密度。根据ASTMG102-1989计算样品的腐蚀速率[24]。电化学阻抗谱(EIS)的测试范围为2 × 105~0.01 Hz。利用ZSimpwin软件对测得的EIS结果进行拟合,每组样品均重复4次。
1.5 浸泡降解实验
将研磨、清洗后的样品浸泡在(37 ± 0.5)℃的Hank's溶液中,分别在7、14和28 d后取出部分样品,对样品的降解行为进行研究。每组样品设4个平行样,分别浸泡在40 mL的Hank's溶液中。样品取出后,用去离子水清洗样品表面,吹干并置于真空干燥箱中。通过DSX100体视显微镜和SEM对样品腐蚀产物的形貌进行表征。根据标准ASTM G1-2003,将样品浸泡在80℃、200 mg/mL的CrO3溶液中1 min以去除腐蚀产物,通过体视显微镜和SEM对去除腐蚀产物后的样品表面形貌进行表征。记录浸泡前的样品重量与去除腐蚀产物之后的重量,根据标准ASTM G31-1972[25],腐蚀速率的计算公式如下:
式中,V代表腐蚀速率(μm/a);K是常数(8.76 × 104);W是样品失重(g);A是样品暴露面积(cm2);T是浸泡时间(h);D是密度(g/cm3)。
2 实验结果与讨论
2.1 微观组织和相组成
图1是热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金微观组织的OM和SEM像。可以看出,加入Cu元素后,基体相中可明显观察到第二相颗粒的出现。此外,在第二相颗粒周围的Zn基体相(图中深色区域)晶粒尺寸显著小于远离第二相颗粒的Zn基体相。研究[17,26]表明,在加工变形过程中,硬质第二相颗粒附近会形成剧烈变形区,可以促进Zn基体相的动态再结晶过程,起到进一步细化晶粒的作用。加入Zr元素后,可以观察到Zn-2Cu-0.5Zr合金中生成了尺寸较大(10~20 μm),且具有规则形状的新第二相颗粒。由于基体内第二相颗粒数量的增多,第二相颗粒周围的剧烈变形区也随之增多,Zn基体晶粒进一步细化。
图1
图1
热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金的OM和SEM像
Fig.1
OM (a-c) and SEM (d-f) images of hot-extruded Zn (a, d), Zn-2Cu (b, e), and Zn-2Cu-0.5Zr (c, f) alloys (Insets in Figs.1d-f show the high magnified images)
热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金的XRD谱如图2所示。结果表明,Zn-2Cu合金主要由Zn基体相和CuZn5相组成。因此,Zn-2Cu合金表面微观组织中观察到的第二相颗粒应为CuZn5相。此外,在加入Zr元素后,Zn-2Cu-0.5Zr合金的XRD谱中出现了Zn22Zr相的峰,应与微观组织中较大尺寸的第二相颗粒对应。值得注意的是,与热挤压Zn相比,热挤压Zn-2Cu和Zn-2Cu-0.5Zr合金的Zn基体相衍射峰均发生了不同程度的偏移。根据Zn-Cu二元相图和相关报道[27,28],425℃下Cu在Zn中的溶解度为1.7% (质量分数),并且在Zn-1.53Cu合金中没有观察到沉淀相,证明室温下Cu在Zn基体中具有较高固溶度。因此Zn基体相衍射峰的偏移主要是由于Cu的固溶引起的。而Zr在Zn中的溶解度较低,主要以二相形式存在。
图2
图2
热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金的XRD谱
Fig.2
XRD spectra of the hot-extruded Zn, Zn-2Cu, and Zn-2Cu-0.5Zr alloys
2.2 力学性能
图3是热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金在室温下的力学性能结果。可以看出,热挤压Zn的屈服强度、抗拉强度和断后伸长率最低,分别为59 MPa、106 MPa和24%。与热挤压Zn相比,热挤压Zn-2Cu合金的综合力学性能显著提高,屈服强度、抗拉强度和断后伸长率分别达到了162 MPa、193 MPa和54%,表明Cu对Zn的强度和韧性均有提升作用。一方面,CuZn5相颗粒的存在能够阻碍位错的移动,提高合金的抗拉强度;另一方面,Cu元素的加入对Zn基体相同时起到了固溶强化和细晶强化的作用,显著提高了材料的抗拉强度和延展性。加入Zr元素后,合金的综合力学性能进一步增强,屈服强度、抗拉强度和断后伸长率分别提高至192 MPa、213 MPa和61%。这主要是由于Zr元素本身具有细化Zn基体相的作用;同时,Zn22Zr相对合金中位错移动的阻碍作用进一步加强并使Zn基体相在挤压过程中得到进一步细化,从而使得合金的强度与韧性进一步增强。
图3
图3
热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金的力学性能
Fig.3
Mechanical properties of the hot-extruded Zn, Zn-2Cu, and Zn-2Cu-0.5Zr alloys
(a) representative stress-strain curves
(b) calculated yield strength (YS), ultimate tensile strength (UTS) and elongation at fracture
2.3 电化学腐蚀
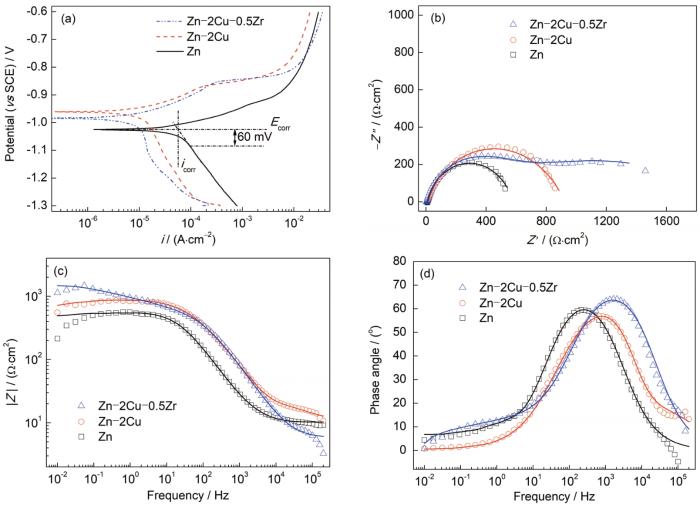
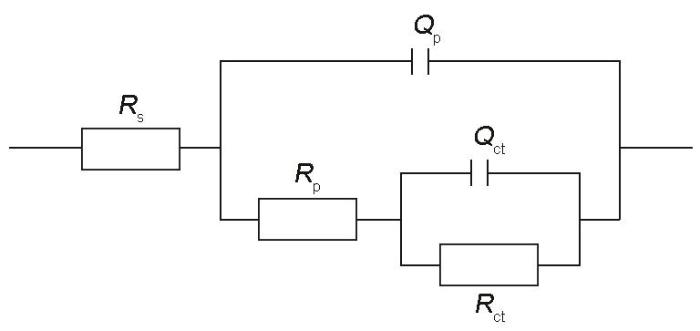
在(37 ± 0.5)℃下Hank's溶液中测定的电化学腐蚀结果如图4所示,计算和拟合结果详见表1。根据动电位极化测量结果(图4a),热挤压Zn-2Cu和Zn-2Cu-0.5Zr合金的腐蚀电位(Ecorr (vs SCE))分别为-0.96和-0.98 V,高于热挤压Zn的腐蚀电位(-1.03 V)。更高的腐蚀电位往往意味着更高的热力学稳定性,因此热挤压Zn-2Cu和Zn-2Cu-0.5Zr合金相较于热挤压纯Zn具有更低的腐蚀倾向。通过Tafel外推法得出,热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金的腐蚀电流密度(icorr)分别为57.22、13.11和10.24 μA/cm2,所对应腐蚀速率分别为0.85、0.20和0.15 mm/a。这表明相较于热挤压Zn,热挤压Zn-2Cu和Zn-2Cu-0.5Zr合金具有更高的抗腐蚀能力。从Nyquist图(图4b)中可以看出,添加Cu和Zr后,热挤压锌基合金样品的阻抗环均有不同程度的增大,这也可以从Bode图(图4c)看出相同趋势。根据Bode相位角图(图4d),所有样品在中频区域均出现宽域峰,应由2个不同时间常数的常相元峰交叠而成。因此,采用Rs(Qp(Rp(QctRct)))等效电路图(如图5所示)对阻抗图谱进行拟合分析,其中Rs代表溶液电阻;Qp和Rp分别表示样品表面腐蚀产物的电容和电阻;Qct和Rct分别代表界面电荷转移反应的双电层电容和电阻。Rct直接与基体/电解液界面处的反应(主要为腐蚀阳极溶解)快慢有关,因此其大小通常与样品的抗腐蚀能力对应。根据拟合结果,热挤压Zn-2Cu-0.5Zr合金具有最高的Rct (1.54 kΩ·cm2),表明其耐腐蚀能力最强。热挤压Zn-2Cu合金的Rct (0.68 kΩ·cm2)低于热挤压Zn-2Cu-0.5Zr,但仍显著高于Zn (0.56 kΩ·cm2)。热挤压Zn-2Cu和Zn-2Cu-0.5Zr合金抗腐蚀性能的提高主要由于基体相的晶粒细化所致。基体相晶粒尺寸越小,由晶体内部与晶界间形成的微腐蚀电池尺度越小、数目越多,更易促进均匀致密腐蚀产物的快速形成与更全面覆盖[29],进而起到对基体的保护作用,这可以由热挤压Zn-2Cu和Zn-2Cu-0.5Zr合金相较于热挤压Zn具有更高的Rp得到验证。
图4
图4
(37 ± 0.5)℃下热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金在Hank's溶液中的电化学测试结果
Fig.4
Potentiodynamic polarization (PDP) curves (a), Nyquist diagrams (b), Bode-impedance diagrams (c), and Bode-phase angle diagrams (d) of the hot-extruded Zn, Zn-2Cu, and Zn-2Cu-0.5Zr alloys in Hank's solution at (37 ± 0.5)oC (Ecorr—corrosion potential, icorr—corrosion current density, i—galvanic current density, Z"—imaginary part of impedance, Z'—real part of impedance, |Z|—impedance modulus)
表1 动电位极化(PDP)曲线和电化学阻抗谱(EIS)的拟合结果
Table 1
| Sample | Ecorr (vs SCE) | icorr | Rs | Qp | Rp | Qct | Rct |
|---|---|---|---|---|---|---|---|
| V | μA·cm-2 | Ω·cm2 | 10-6 S n ·Ω-1·cm-2 | Ω·cm2 | 10-6 S n ·Ω-1·cm-2 | kΩ·cm2 | |
| Zn | -1.03 | 57.22 | 10.27 | 3.59 | 37.77 | 0.74 | 0.56 |
| Zn-2Cu | -0.96 | 13.11 | 10.81 | 19.12 | 225.48 | 14.15 | 0.68 |
| Zn-2Cu-0.5Zr | -0.98 | 10.24 | 10.65 | 8.18 | 433.34 | 65.43 | 1.54 |
图5
2.4 长期浸泡腐蚀评价
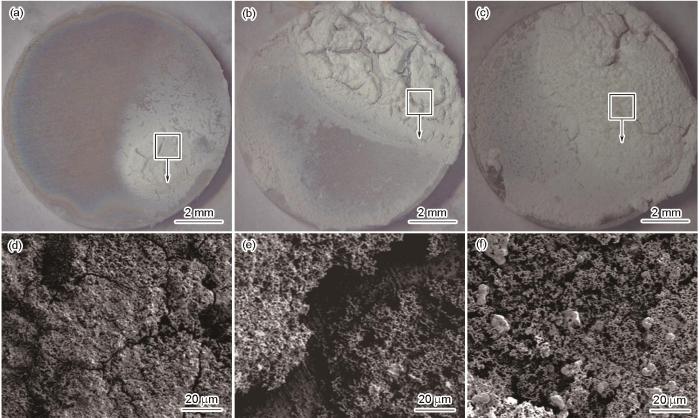
图6~8分别为热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金在Hank's溶液中浸泡不同时间后的表面形貌。从体式显微镜照片可以看出,浸泡7 d后,所有样品表面均出现了局部的腐蚀产物堆积区域。其中,热挤压Zn表面腐蚀产物堆积最为明显,而热挤压Zn-2Cu-0.5Zr样品表面腐蚀产物形成最不明显。随着浸泡时间的延长,热挤压Zn表面腐蚀产物覆盖面积明显扩大,且腐蚀产物间的不连续缺陷区较多。热挤压Zn-2Cu合金展现出与Zn类似的规律,且在浸泡28 d后腐蚀产物覆盖面积甚至高于热挤压Zn。热挤压Zn-2Cu-0.5Zr合金的腐蚀产物覆盖区域在各个时间点均最不明显。从SEM像可以看出,所有样品在Hank's溶液中浸泡后的表面腐蚀产物主要呈球形颗粒状。值得注意的是,热挤压Zn和Zn-2Cu合金表面腐蚀产物较为松散,可以观察到明显的缝隙状不连续区域。浸泡过程中溶液更易通过这些不连续缝隙区域到达基体表面,加速局部腐蚀。比较图6~8可以看出,热挤压Zn-2Cu-0.5Zr合金的表面腐蚀产物最致密且覆盖更为完整,能够有效阻隔溶液与基体的接触,进而起到对基体的保护作用。
图6
图6
(37 ± 0.5)℃下热挤压Zn在Hank's溶液中浸泡不同时间后的表面形貌
Fig.6
Low (a-c) and locally high (d-f) magnified surface morphologies of hot-extruded Zn immersed in Hank's solution at (37 ± 0.5)oC for 7 d (a, d), 14 d (b, e), and 28 d (c, f)
图7
图7
(37 ± 0.5)℃下热挤压Zn-2Cu合金在Hank's溶液中浸泡不同时间后的表面形貌
Fig.7
Low (a-c) and locally high (d-f) magnified surface morphologies of hot-extruded Zn-2Cu alloy immersed in Hank's solution at (37 ± 0.5)oC for 7 d (a, d), 14 d (b, e), and 28 d (c, f)
图8
图8
(37 ± 0.5)℃下热挤压Zn-2Cu-0.5Zr合金在Hank's溶液中浸泡不同时间后的表面形貌
Fig.8
Low (a-c) and locally high (d-f) magnified surface morphologies of hot-extruded Zn-2Cu-0.5Zr alloy immersed in Hank's solution at (37 ± 0.5)oC for 7 d (a, d), 14 d (b, e), and 28 d (c, f)
图9是热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金在Hank's溶液中浸泡不同时间并去除表面腐蚀产物之后的样品表面形貌。从体式显微镜照片可以看出,所有样品在浸泡7 d后均出现了局部腐蚀位点,随着浸泡时间的延长,腐蚀区域面积不断扩大。在浸泡7~14 d时,热挤压Zn表面腐蚀区域面积迅速增加,在浸泡28 d后,表面出现大量新的腐蚀位点,表面破坏严重。热挤压Zn-2Cu样品的腐蚀趋势与热挤压Zn类似,但在浸泡28 d后,其表面破坏更为严重,且扩展至整个表面,这表明热挤压Zn-2Cu合金的长期降解行为最差。热挤压Zn和Zn-2Cu合金浸泡28 d后的样品表面腐蚀区域存在大量由点蚀纵深发展的小孔腐蚀,这可能极易导致材料的提前力学失效。相反,热挤压Zn-2Cu-0.5Zr合金表面的腐蚀区域面积在整个浸泡周期内均弱于热挤压Zn和Zn-2Cu合金,腐蚀更为均匀,也没有明显的小孔腐蚀,展现出更好的长期降解模式。
图9
图9
(37 ± 0.5)℃下热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金在Hank's溶液中浸泡不同时间后去除腐蚀产物的表面形貌
Fig.9
Surface morphologies of hot-extruded Zn (a, d, g), Zn-2Cu (b, e, h), and Zn-2Cu-0.5Zr (c, f, i) alloys immersed in Hank's solution at (37 ± 0.5)oC for 7 d (a-c), 14 d (d-f), and 28 d (g-i) after removal of corrosion products, and locally high magnified SEM images of Figs.9g-i (j-l)
图10是通过失重法测定浸泡后的腐蚀速率。热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金的腐蚀速率分别为140~160、120~160和70~100 μm/a。其中,热挤压Zn的腐蚀速率在整个浸泡周期内较为稳定。热挤压Zn-2Cu合金在浸泡7和14 d后腐蚀速率略低于纯Zn,但在浸泡28 d后其腐蚀速率迅速增加,甚至高于热挤压纯Zn。结合腐蚀形貌可知,热挤压Zn-2Cu合金在浸泡14 d后基体腐蚀扩展加速,这可能是由于表面腐蚀产物层被基体相与第二相间形成的电偶腐蚀破坏所造成的。但是,热挤压Zn-2Cu-0.5Zr合金的腐蚀速率明显低于热挤压Zn和Zn-2Cu合金,主要原因归结如下:虽然热挤压Zn-2Cu-0.5Zr合金中仍存以上类似的第二相,但其基体相对热挤压Zn和Zn-2Cu合金晶粒更为细小,这使得腐蚀过程所形成的腐蚀产物覆盖更为完整和均匀致密,因而不但更有效地阻止了溶液与金属基底的接触,减缓了对金属基体的腐蚀,而且也在一定程度上避免或减弱了CuZn5和Zn22Zr第二相与基底(被覆盖保护更为充分)间所形成的电偶腐蚀,从而从整体来说腐蚀更为缓慢,并且腐蚀形态也更为均匀。
图10
图10
失重法测量(37 ± 0.5)℃下热挤压Zn、Zn-2Cu和Zn-2Cu-0.5Zr合金在Hank's溶液中浸泡不同时间的腐蚀速率
Fig.10
Corrosion rates calculated from weight loss of hot-extruded Zn, Zn-2Cu, and Zn-2Cu-0.5Zr alloys after immersion in Hank's solution at (37 ± 0.5)oC for different time
2.5 热挤压Zn-2Cu-0.5Zr合金的性能改善机制
图11为热挤压Zn-2Cu-0.5Zr合金的性能改善机制示意图,从“材料工艺-结构-性能”的关系讨论其内在机理。金属材料的力学性能取决于其微观组织[30],从实验结果来看,热挤压后的Zn晶粒尺寸仍保持较大,细化晶粒作用有限。加入合金元素Cu后,部分Cu元素与基体形成固溶体,在塑性变形过程中,能够对位错起到一定的钉扎作用,进而提高力学强度。另一部分Cu元素以CuZn5相形式存在。CuZn5相的存在对提高材料综合力学性能的作用如下:一方面其弥散分布能够有效阻碍滑移过程中的位错运动,提高塑性变形抗力;另一方面,在热挤压过程中,CuZn5可以作为硬质第二相颗粒促进周围Zn基体的动态再结晶并促使晶粒细化[17]。多晶材料的块体变形是所有晶粒的变形协调的总和,晶粒越细,相同体积内的晶粒数量越多,变形分散到更多晶粒中进行,使材料能够在断裂前承受更大的变形量,从而提高材料的塑性。此外,晶粒越细,晶界面积越大,晶界越曲折,对位错滑移的阻碍作用越大,能够有效抑制裂纹的扩展,进而提高材料的强度。Zr在Zn基体中几乎没有固溶度,主要以Zn22Zr相形式存在。合金元素Zr的加入,使基体中弥散分布的硬质第二相颗粒数量增多,通过增强对滑移过程中位错运动的阻碍作用,以及基体的动态再结晶过程,促使晶粒细化。同样,非连续的Zn22Zr相颗粒也有一定的阻止裂纹扩展的能力[31]。综上,细晶强/韧化和第二相弥散强化是热挤压Zn-2Cu-0.5Zr合金具备综合优良力学性能的根本原因。
图11
图11
热挤压Zn-2Cu-0.5Zr合金性能改善机制示意图
Fig.11
Schematic of the improvement mechanisms for the enhanced mechanical properties and degradation behavior of hot-extruded Zn-2Cu-0.5Zr alloy
材料的微观组织对锌基金属材料的降解行为同样具有重要影响。Zn基体晶粒粗大,表面生成的腐蚀产物疏松多孔,难以完全覆盖Zn基体,Hank's溶液通过腐蚀产物层缺陷处与基体直接接触,促进了基体的腐蚀,并会加速形成局部腐蚀原电池[32]。合金元素Cu的加入虽有利于基体相的晶粒细化,这有利于其抗腐蚀能力提高,但固溶的Cu可能会加速Zn基体自身的腐蚀原电池形成,从而加速腐蚀;同样,CuZn5相也可能倾向与Zn基体形成腐蚀原电池而加速腐蚀。这种腐蚀加剧在添加更高含量Cu的合金中更为明显[28],印证了以上推测。此外,以上局部腐蚀也更易随着浸泡时间的延长而呈现出腐蚀加剧的情况,如由点蚀发展成纵深发展的小孔腐蚀,而这对于可降解金属来讲必须尽力避免,否则可能造成提前的力学支撑失效[33]。加入Zr元素后,基体相进一步细化,使得表面生成的腐蚀产物层更为均匀致密,覆盖也更为全面而完整,对基体的保护作用提高,局部腐蚀加剧的情况也得以避免,从而使得合金的长期降解行为更加缓慢和均匀。
综上所述,热挤压Zn-2Cu-0.5Zr合金具有良好的综合力学性能和更均匀的降解行为,展示了作为生物可降解金属植入物材料的潜力。
3 结论
(1) 加入合金元素Cu后,Zn基体中出现了CuZn5相。在热挤压过程中,CuZn5相促进了周围Zn基体的动态再结晶过程,显著细化晶粒。合金元素Zr的加入进一步细化了基体相晶粒,并在基体中形成了Zn22Zr相,显著提高了合金的强度和韧性。其中,热挤压Zn-2Cu-0.5Zr合金的抗拉强度达到213 MPa,是热挤压Zn的2倍,断后伸长率则提升至61%。
(2) Zr元素的加入显著降低了合金的腐蚀速率,并改善了合金的降解模式。这主要归功于Zr元素对基体相晶粒的细化作用,更小的晶粒尺寸促使表面生成的腐蚀产物层更加均匀致密,进而有效隔绝了电解液对基体的侵蚀。
参考文献
Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents
[J].
Zinc-based alloys for degradable vascular stent applications
[J].The search for biodegradable metals with mechanical properties equal or higher to those of currently used permanent biomaterials, such as stainless steels, cobalt chromium and titanium alloys, desirable in vivo degradation rate and uniform corrosion is still an open challenge. Magnesium (Mg), iron (Fe) and zinc (Zn)-based alloys have been proposed as biodegradable metals for medical applications. Over the last two decades, extensive research has been done on Mg and Fe. Fe-based alloys show appropriate mechanical properties, but their degradation rate is an order of magnitude below the benchmark value. In comparison, alongside the insufficient mechanical performance of most of its alloys, Mg degradation rate has proven to be too high in a physiological environment and corrosion is rarely uniform. During the last few years, Zn alloys have been explored by the biomedical community as potential materials for bioabsorbable vascular stents due to their tolerable corrosion rates and tunable mechanical properties. This review summarizes recent progress made in developing Zn alloys for vascular stenting application. Novel Zn alloys are discussed regarding their microstructural characteristics, mechanical properties, corrosion behavior and in vivo performance.Numerous studies on magnesium and iron materials have been reported to date, in an effort to formulate bioabsorbable stents with tailorable mechanical characteristics and corrosion behavior. Crucial concerns regarding poor ductility and remarkably rapid corrosion of magnesium, and very slow degradation of iron, seem to be still not desirably fulfilled. Zinc was introduced as a potential implant material in 2013 due to its promising biodegradability and biocompatibility. Since then, extensive investigations have been made toward development of zinc alloys that meet clinical benchmarks for vascular scaffolding. This review critically surveys the zinc alloys developed since 2013 from metallurgical and biodegradation points of view. Microstructural features, mechanical, corrosion and in vivo performances of these new alloys are thoroughly reviewed and evaluated.Copyright © 2018 Acta Materialia Inc. All rights reserved.
Biodegradable metals for cardiovascular stents: From clinical concerns to recent Zn-alloys
[J].
Trace elements in human physiology and pathology: Zinc and metallothioneins
[J].Zinc is one of the most abundant nutritionally essential elements in the human body. It is found in all body tissues with 85% of the whole body zinc in muscle and bone, 11% in the skin and the liver and the remaining in all the other tissues. In multicellular organisms, virtually all zinc is intracellular, 30-40% is located in the nucleus, 50% in the cytoplasm, organelles and specialized vesicles (for digestive enzymes or hormone storage) and the remainder in the cell membrane. Zinc intake ranges from 107 to 231 micromol/d depending on the source, and human zinc requirement is estimated at 15 mg/d. Zinc has been shown to be essential to the structure and function of a large number of macromolecules and for over 300 enzymic reactions. It has both catalytic and structural roles in enzymes, while in zinc finger motifs, it provides a scaffold that organizes protein sub-domains for the interaction with either DNA or other proteins. It is critical for the function of a number of metalloproteins, inducing members of oxido-reductase, hydrolase ligase, lyase family and has co-activating functions with copper in superoxide dismutase or phospholipase C. The zinc ion (Zn(++)) does not participate in redox reactions, which makes it a stable ion in a biological medium whose potential is in constant flux. Zinc ions are hydrophilic and do not cross cell membranes by passive diffusion. In general, transport has been described as having both saturable and non-saturable components, depending on the Zn(II) concentrations involved. Zinc ions exist primarily in the form of complexes with proteins and nucleic acids and participate in all aspects of intermediary metabolism, transmission and regulation of the expression of genetic information, storage, synthesis and action of peptide hormones and structural maintenance of chromatin and biomembranes.
Zinc-based biomaterials for regeneration and therapy
[J].
Research progress on biodegradable zinc-based biomaterials
[J].
可降解锌基生物材料的研究进展
[J].
In vitro and in vivo studies of Zn-Mn biodegradable metals designed for orthopedic applications
[J].In recent years, Zn-based materials provide a new option as biodegradable metals for orthopedic applications. To improve the low strength and brittle nature of pure Zn, small amounts of alloying element Mn (0.1, 0.4 and 0.8 wt.%) were added into Zn to fabricate binary Zn-Mn alloys. An extremely high elongation (83.96 ± 2.36%) was achieved in the resulting Zn-0.8 wt.%Mn alloy. Moreover, Zn-Mn alloys displayed significantly improved cytocompatibility as compared to pure Zn, according to cell proliferation and morphology analyses. More importantly, a significantly improved osteogenic activity was verified after adding Mn regarding ALP activity and osteogenic expression. Furthermore, Zn-0.8 wt.%Mn alloy scaffolds were implanted into the rat femoral condyle for repairing bone defects with pure Ti as control. Enhanced osteogenic activities were confirmed for Zn-0.8Mn alloy in contrast to pure Ti based on Micro-CT and histological results, and favorable in vivo biosafety of Zn-0.8Mn alloy was verified by H&E staining and blood tests. The exceptional mechanical performance and favorable osteogenic capability render Zn-Mn alloy a promising candidate material in the treatment of bone defects or fracture repair. STATEMENT OF SIGNIFICANCE: The element Mn, on the one hand, as an essential trace element in the human body, promotes cell proliferation, adhesion, spreading, and regulates bone metabolism; on the other hand, it could significantly improve the ductility of Zn alloys. Here, we systematically reported the biocompatibility and biofunctionality of binary biodegradable Zn-Mn alloys in the bone environment. The Zn-Mn alloys promoted MC3T3-E1 cell proliferation, adhesion, spreading, and osteogenic differentiation in vitro. Furthermore, a rat femoral condyle defect model was established; porous Zn-Mn alloy scaffolds were manufactured to repair the bone defects. Significant bone regenerations, considerable bone ingrowth, and desirable biosafety were confirmed in vivo. Therefore, biodegradable Zn-Mn with promising osteogenic properties may become new options for orthopedic implant materials.Copyright © 2020. Published by Elsevier Ltd.
Zinc as a therapeutic agent in bone regeneration
[J].
Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation
[J].In the present work Zn-Mg alloys containing up to 3wt.% Mg were studied as potential biodegradable materials for medical use. The structure, mechanical properties and corrosion behavior of these alloys were investigated and compared with those of pure Mg, AZ91HP and casting Zn-Al-Cu alloys. The structures were examined by light and scanning electron microscopy (SEM), and tensile and hardness testing were used to characterize the mechanical properties of the alloys. The corrosion behavior of the materials in simulated body fluid with pH values of 5, 7 and 10 was determined by immersion tests, potentiodynamic measurements and by monitoring the pH value evolution during corrosion. The surfaces of the corroded alloys were investigated by SEM, energy-dispersive spectrometry and X-ray photoelectron spectroscopy. It was found that a maximum strength and elongation of 150MPa and 2%, respectively, were achieved at Mg contents of approximately 1wt.%. These mechanical properties are discussed in relation to the structural features of the alloys. The corrosion rates of the Zn-Mg alloys were determined to be significantly lower than those of Mg and AZ91HP alloys. The former alloys corroded at rates of the order of tens of microns per year, whereas the corrosion rates of the latter were of the order of hundreds of microns per year. Possible zinc doses and toxicity were estimated from the corrosion behavior of the zinc alloys. It was found that these doses are negligible compared with the tolerable biological daily limit of zinc.Copyright © 2011 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation
[J]. J.
Research progress in biodegradable metals for stent application
[J].
血管支架用可降解金属研究进展
[J].在过去的20年间,随着对可降解金属研究的不断深入,从合金成分设计到熔炼制造加工,从毛细管到支架激光加工和药物涂覆等相关技术不断成熟。可降解金属支架也从一个概念发展为实际产品,并形成3个材料体系分支:可降解镁合金支架已经开展了大量的动物实验和临床实验,结果显示其良好的表现和临床安全性,Biotronik公司生产的可降解镁合金产品Magmaris在2016年获得CE认证;可降解铁合金支架目前处在动物实验阶段,注氮铁合金支架具有优异的力学性能,动物实验结果显示注氮铁支架具有良好的生物相容性;可降解锌合金支架近几年才得到人们的关注,目前的体内动物实验研究结果显示,纯Zn丝在小鼠体内具有良好的降解性能和生物相容性,暂未见体内支架研究报道。本文在综合评述可降解金属支架材料的研究现状基础上,展望了可降解金属支架在性能优化、药物洗脱和智能化方面的未来发展趋势。
Comparative corrosion behavior of Zn with Fe and Mg in the course of immersion degradation in phosphate buffered saline
[J].
Effects of Sr addition on microstructure, mechanical properties and in vitro degradation behavior of as-extruded Zn-Sr binary alloys
[J].
Role of Cu element in biomedical metal alloy design
[J].
Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications
[J]. J.
Investigation of zinc-copper alloys as potential materials for craniomaxillofacial osteosynthesis implants
[J].
Biodegradable Zn-Cu alloys show antibacterial activity against MRSA bone infection by inhibiting pathogen adhesion and biofilm formation
[J].
Alloying design of biodegradable zinc as promising bone implants for load-bearing applications
[J].
The effect of zirconium addition on corrosion behavior of Zn-Zr alloys as biodegradable orthopedic implant application
[J].
The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review
[J].Zinc has been identified as one of the most promising biodegradable metals along with magnesium and iron. Zinc appears to address some of the core engineering problems associated with magnesium and iron when applied to biomedical implant applications; hence the increase in the amount of research investigations on the metal in the last few years. In this review, the current state-of-the-art on biodegradable Zn, including recent developments, current opportunities and future directions of research are discussed. The discussions are presented with a specific focus on reviewing the relationships that exist between mechanical properties, biodegradability, and biocompatibility of zinc with alloying and fabrication techniques. This work hopes to guide future studies on biodegradable Zn that will help in advancing this field of research. STATEMENT OF SIGNIFICANCE: (i) The review offers an up-to-date and comprehensive review of the influence of alloying and fabrication technique on mechanical properties, biodegradability and biocompatibility of Zn; (ii) the work cites the most relevant biodegradable Zn fabrication processes including additive manufacturing techniques; (iii) the review includes a listing of research gap and future research directions for the field of biodegradable Zn.Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr
[J].
Controllable biodegradation and enhanced osseointegration of ZrO2-nanofilm coated Zn-Li alloy: In vitro and in vivo studies
[J].Zinc and its alloys have emerged as a new research direction of biodegradable metals (BMs) due to the significant physiological functions of Zn ions in human body. However, low inhibitory concentration threshold value to cause cytotoxicity by Zn ions during in vitro study and delayed osseointegration in vivo are two key flaws for the bulk Zn-based BMs. To combat these issues, we constructed a barrier layer of ZrO nanofilm on the surface of Zn-0.1(wt.%) Li alloy via atomic layer deposition (ALD). A decreased release of Zn ions accompanied with accelerated release of Li ions was observed on account of galvanic coupling between the coating compositions and Zn-0.1Li alloy substrate. Cytocompatibility assay reflected that ZrO nanofilm coated Zn-0.1Li alloy exhibited improved cell adhesion and viability. Histological analysis also demonstrated better in vivo osseointegration for the ZrO nanofilm coated Zn-0.1Li alloy. Hence, the present study elucidated that the ALD of ZrO nanofilm on Zn-based BMs can effectively promote osseointegration and control their biodegradation behavior. STATEMENT OF SIGNIFICANCE: Zn-Li binary alloy was reported recently to be the promising biodegradable metals with ultimate tensile strength over 500 MPa, yet the low inhibitory concentration threshold value to cause cytotoxicity by Zn ions is the obstacle needed to be overcome. As a pilot study, a systematic investigation on the ZrO nanofilm coated Zn-Li alloy, prepared by atomic layer deposition (ALD) technique, was conducted in the present study, which involved in the formation process, in vitro and in vivo degradation behavior as well as biocompatibility evaluation. We found a controllable corrosion rate and better in vivo osseointegration can be achieved by ZrO nanofilm coating on Zn-Li alloy, which provides new insight into the surface modification on biodegradable Zn alloys for usage within bone.Copyright © 2020 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium
[J].Rare earth elements are promising alloying element candidates for magnesium alloys used as biodegradable devices in biomedical applications. Rare earth elements have significant effects on the high temperature strength as well as the creep resistance of alloys and they improve magnesium corrosion resistance. We focused on lanthanum, neodymium and cerium to produce magnesium alloys with commonly used rare earth element concentrations. We showed that low concentrations of rare earth elements do not promote bone growth inside a 750 μm broad area around the implant. However, increased bone growth was observed at a greater distance from the degrading alloys. Clinically and histologically, the alloys and their corrosion products caused no systematic or local cytotoxicological effects. Using microtomography and in vitro experiments, we could show that the magnesium-rare earth element alloys showed low corrosion rates, both in in vitro and in vivo. The lanthanum- and cerium-containing alloys degraded at comparable rates, whereas the neodymium-containing alloy showed the lowest corrosion rates.Copyright © 2014 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Appropriately adapted properties of hot-extruded Zn-0.5Cu-xFe alloys aimed for biodegradable guided bone regeneration membrane application
[J].
Effects of extrusion and heat treatment on the mechanical properties and biocorrosion behaviors of a Mg-Nd-Zn-Zr alloy
[J]. J.
Unidirectional solidification of Zn-rich Zn-Cu peritectic alloys—I. Microstructure selection
[J].
Effects of extrusion temperature on microstructure, mechanical properties and in vitro degradation behavior of biodegradable Zn-3Cu-0.5Fe alloy
[J].
Ultrafine- and uniform-grained biodegradable Zn-0.5Mn alloy: grain refinement mechanism, corrosion behavior, and biocompatibility in vivo
[J].
Review of microstructures and properties of zinc alloys
[J].
Influence of secondary phases on crack initiation and propagation during fracture process of as-cast Mg-Al-Zn-Nd alloy
[J].
Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn-1.5Mg alloy
[J]. J.