碳钢作为基础设施结构中用量最大的金属材料,其在使用过程中不可避免地遇到大气腐蚀问题。而由腐蚀导致的工程装备、关键结构以及基础设施损坏十分严重,进而引发灾难性事故,给国民经济带来巨大的损失。因而,碳钢的大气腐蚀与防护问题一直受到学者们的广泛关注。众所周知,碳钢的大气腐蚀实际上是钢基体与周围的大气环境之间发生化学或电化学反应而失效的过程。科研人员[1~8]对碳钢在多种实际大气环境中的腐蚀问题开展了大量研究。碳钢在清洁的乡村大气环境腐蚀较慢,在SO2含量较高的城市工业大气环境中,受表面锈层的结构和产物组成的影响,腐蚀过程加剧[5,9~12]。在海洋大气环境中,空气中大量的Cl-对锈层的破坏作用较强,使钢表面很难形成稳定的保护性锈层,从而加速腐蚀过程的进行[3,8,13~15]。在盐湖大气环境中,空气中大量的Mg2+富集在内锈层中,对碳钢的腐蚀过程具有重要的影响[16,17]。由于不同地域的气候环境中的温度、相对湿度、污染物组成及含量的差异性很大,材料的腐蚀速率和腐蚀过程也有很大的差别。即使同样是海洋大气环境,锈层的结构也有明显的差异[8,13~15,18]。
在推进远海岛屿建设的过程中,人们发现现有远海岛屿上的基础设施材料以及部署在该海域附近岛屿上的战机的大气腐蚀问题极其严重,连常用的电器也比其他地区的腐蚀问题严重,而造成这些问题的原因尚不明确。虽然已有一些学者[19,20]对材料在类似环境(高温、高湿、高盐和高辐照)的南美地区的大气腐蚀行为进行了研究,但在地处中南亚的南沙地区材料的腐蚀数据非常少。此外,在对户外长期暴晒数据进行分析时,大多用朝天面的腐蚀情况来代表整体腐蚀,这样显然是不够严谨的。而目前关于朝天面和朝地面锈层结构的差异及其随时间变化的研究更少。因此,开展碳钢在南沙热带海洋大气环境下的腐蚀数据的积累和腐蚀规律的研究,不仅对环境的腐蚀性评价和了解装备在南沙地区环境中的使用寿命具有重要的参考价值,而且对科学研究和国防建设都具有重要意义。
本工作研究了低碳钢Q235和耐候钢Q450NQR1在南沙群岛地区暴晒34个月后的腐蚀行为,着重探讨了2种碳钢朝天面和朝地面锈层对腐蚀动力学变化规律、锈层结构和腐蚀机理的影响。
1 实验方法
1.1 实验材料
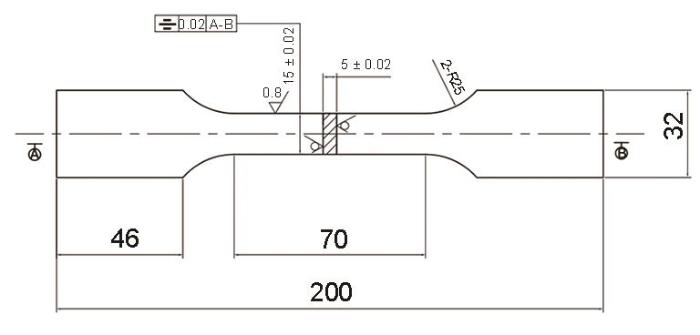
户外暴晒实验所用材料为Q235低碳钢和Q450NQR1耐候钢,2种钢的主要化学成分见表1。按照GB/T 14165-2008将试样加工为200 mm × 100 mm × 5.5 mm,每个周期4片,其中3片平行样用来失重分析,1片作为锈层产物形貌、结构和成分分析。按照GB/T 228—2010将试样加工成如图1所示的力学拉伸件。所有样品经机加工打磨至表面粗糙度(Ra)为0.8,实验前所有试样经丙酮除油、酒精清洗后吹干备用。用于失重分析的试样在干燥器中贮存24 h后,用精度为0.01 g的天平进行称重。用于电化学测量的试样的制备方法为:首先将取回的带锈试样切割成平面尺寸大于10 mm × 10 mm的小块试样,然后用Cu导线与试样的非测试面进行焊接,最后用松香石蜡进行封装,留出约1 cm2的工作面积。
表1 Q235和Q450NQR1钢的化学成分 (mass fraction / %)
Table 1
| Steel | C | Si | Mn | P | S | Cr | Ni | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Q235 | 0.22 | 0.08 | 0.10 | 0.015 | 0.003 | - | - | - | Bal. |
| Q450NQR1 | 0.08 | 0.31 | 0.41 | 0.073 | 0.006 | 0.47 | 0.15 | 0.29 | Bal. |
图1
1.2 户外暴晒实验
暴晒地点位于南沙群岛(9°54′N,115°32′E),暴晒场距离海边约150 m,根据地理位置分布,该地区属于典型的年温差和日温差都小的高湿热、强辐射和高盐雾热带海洋大气。暴晒场周围的年平均温度为28.1℃,年平均相对湿度为81.6%,年平均降雨量为2000 mm,年降雨天数为210 d,Cl-沉降量为11.54 mg/(100 cm2·d)。该地区地处于赤道附近,由于受太阳光直射的影响,夏季地表温度最高可达60℃以上。户外暴晒样品按照GB/T 14162—2008进行实验,即样品正面与水平面成45°进行暴晒,暴晒时间为2017年9月1日至2020年8月3日,取样周期为1、5、12、21和34个月。其中暴晒12个月内的腐蚀机理见文献[21],本工作着重对暴晒21和34个月的试样的腐蚀行为进行分析。
1.3 腐蚀结果的表征
暴晒后试样表面的锈层先用刀片刮取以备成分分析,然后按照GB/T 16545—2015,将3.5 g C6H12N4和500 mL HCl (密度为1.19 g/mL)溶于蒸馏水配制成1 L的除锈液。室温下将带锈试样浸泡在除锈液中,浸泡后用毛刷刷洗残留的产物。待腐蚀产物完全去除后,用蒸馏水清洗,再用酒精二次清洗后吹干,放入干燥器中,干燥24 h后取出称重。
利用数码相机对锈层的宏观形貌进行观察。利用Inspect F50扫描电子显微镜(SEM)及配套的能谱仪(EDS)对锈层的微观形貌和元素分布进行分析。用来进行锈层结构分析的试样用环氧树脂镶嵌,固化后用砂纸依次打磨至2000号,抛光、酒精清洗吹干后,喷碳备用。
将刮取的腐蚀产物在研钵中进行研磨,制成粉末。利用D/max 2500PC型X射线衍射仪(XRD)进行相组成分析,采用Cu靶,电压和电流分别设定为50 kV和3 A。扫描角度为10°~70°,步长设定为0.02°,扫描速率为1°/min。采用Jade软件和Maud软件对锈层的相组成及相对含量进行分析。
电化学测试采用PARSTAT 2273电化学工作站,室温下配合使用传统三电极体系:Pt为辅助电极,饱和甘汞电极(SCE)为参比电极,暴晒不同时间后的带锈试样为工作电极。选用0.1 mol/L的NaCl作为电解液。极化曲线测试过程中扫描速率为20 mV/min,扫描范围为-0.25~0.25 V (vsEOCP,EOCP为开路电位)。
2 实验结果及分析
2.1 Q235和Q450NQR1腐蚀动力学分析
为方便对不同暴晒地区的腐蚀数据进行对比,碳钢在户外大气环境中暴晒后的腐蚀速率常用腐蚀深度来表示,腐蚀深度与试样质量的关系为:
式中,D为腐蚀深度;w0为暴晒前试样的质量;wt为除锈后试样的质量;ρ为碳钢的密度;S为暴晒试样的表面积。
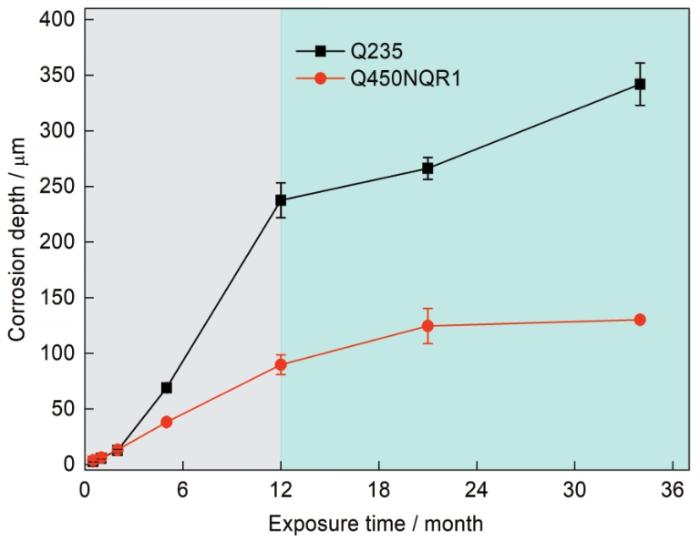
图2为低碳钢Q235和耐候钢Q450NQR1在南沙海洋大气环境中暴晒不同时间后腐蚀深度的变化。2种钢的腐蚀深度均随暴晒时间的延长逐渐增加。这种增加趋势以暴晒12个月时为节点可分为2个阶段:第一阶段腐蚀深度增加较快,第二阶段增速较慢。第一阶段,2种碳钢腐蚀动力学规律符合幂指数关系,幂函数中的指数n都大于1[21];第二阶段,Q235的腐蚀动力学变化仍为腐蚀速率逐渐增大趋势,增速比第一阶段减慢;而Q450NQR1的变化为腐蚀速率逐渐减小的均势。第二阶段腐蚀动力学的变化规律,仍需要更多的腐蚀数据进行拟合才更准确。在已有的研究[7,22]中,碳钢在户外长期暴晒后腐蚀速率也都会出现分段现象。对于耐候钢来说,一般在使用3 a后才形成保护性较强的锈层[23,24],而本研究中,暴晒1 a后的腐蚀速率明显减小。说明在该海洋大气环境中耐候钢可在相对较短的时间内形成保护性锈层。
图2
图2
碳钢Q235和耐候钢Q450NQR1的腐蚀深度随暴晒时间的变化
Fig.2
Variations in the corrosion depth of Q235 and Q450NQR1 as function of exposure time
2.2 锈层成分分析
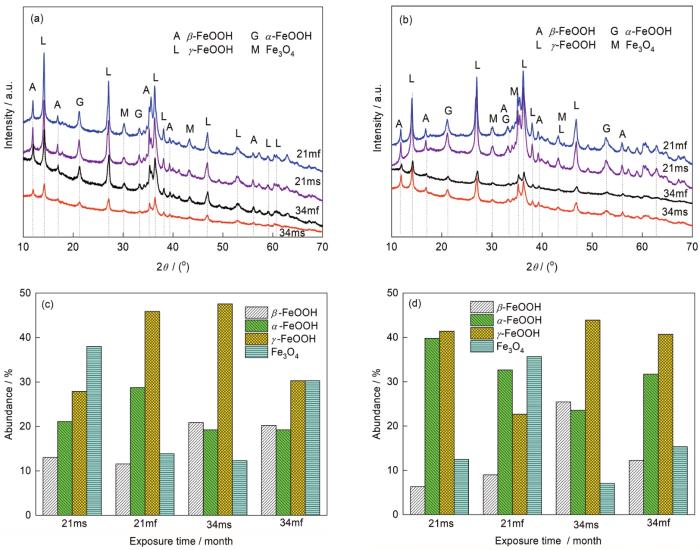
对Q235和Q450NQR1朝天面和朝地面两侧的锈层进行成分分析,得到腐蚀产物组成随暴晒时间的变化情况如图3a和b所示。暴晒21和34个月后,2种钢的朝天面和朝地面锈层组成主要为γ-FeOOH、β-FeOOH、α-FeOOH和Fe3O4。对于Q235来说(图3c),样品两面锈层中β-FeOOH的相对含量随着暴晒时间的延长而增大,而在同一暴晒时间,朝天面和朝地面锈层中β-FeOOH的相对含量相差不大。样品两面锈层中α-FeOOH的相对含量随着暴晒时间的延长而减小,暴晒21个月时,朝天面中α-FeOOH的相对含量比朝地面的少,暴晒34个月时,两面锈层中α-FeOOH的相对含量相近。朝天面和朝地面锈层中的γ-FeOOH和Fe3O4的相对含量在21和34个月时的变化规律相反。对于Q450NQR1来说(图3d),随着暴晒时间从21个月延长到34个月时,样品两面锈层中的α-FeOOH和Fe3O4的相对含量都减小,而β-FeOOH和γ-FeOOH的相对含量则相反,而朝天面和朝地面的4种产物组成都有一定的差异。
图3
图3
Q235和Q450NQR1表面锈层相组成及其相对含量随暴晒时间的变化
Fig.3
Compositions (a, b) and variations of the relative content of each phase (c, d) in rust layer formed on Q235 (a, c) and Q450NQR1 (b, d) as a function of exposure time (21ms—sky-ward side after 21 months exposure, 21mf—field-ward side after 21 months exposure, 34ms—sky-ward side after 34 months exposure, 34mf—field-ward side after 34 months exposure)
2.3 锈层形貌分析
2.3.1 宏观形貌
图4
图4
Q235和Q450NQR1表面锈层的宏观形貌
Fig.4
Macro-morphologies of the rust layer on skyward (a-d) and field-ward (e-h) sides of Q235 and Q450NQR1 (Arrows show local peeling of rust layer)
(a, e) Q235, 21 months (b, f) Q235, 34 months (c, g) Q450NQR1, 21 months (d, h) Q450NQR1, 34 months
2.3.2 截面形貌
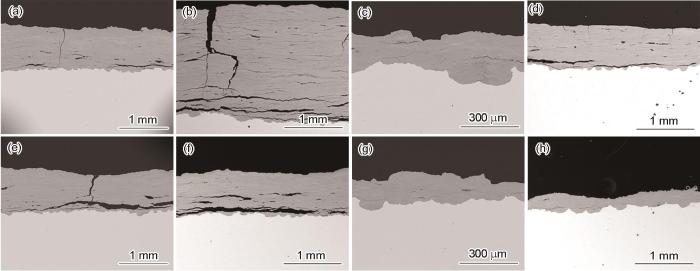
图5为碳钢Q235和耐候钢Q450NQR1暴晒21和34个月后,朝天面和朝地面锈层结构的变化情况。可以看出,暴晒21个月时,低碳钢Q235两面的锈层都较厚,厚度都已经超过了1 mm。到34个月时,朝天面的锈层继续增厚,锈层厚度介于1.79~2.00 mm之间,朝地面锈层的厚度变化不大,说明朝地面锈层随着暴晒时间的延长,在生长的过程中更容易脱落,进一步印证了宏观形貌的结论。从锈层结构上来看,锈层中裂纹大多平行于基体,暴晒21个月时,朝天面和朝地面的锈层中均出现贯穿的纵向裂纹。而在暴晒34个月后,朝天面锈层中的横纵向裂纹的宽度都增大(图5b)。无论是暴晒21个月还是34个月,锈层中从最外层向内穿至基体的纵向裂纹为腐蚀介质和O2的扩散提供了通道,加速了腐蚀过程的进行。此外,在层状锈层的空隙处极易聚积雨水,这些水蒸气在高湿热的大气环境中,很难从较厚的锈层中蒸发[26],潮湿的环境促进了Fe的阳极溶解,以及活性较强的FeOOH的还原反应过程,加速电化学过程。
图5
图5
Q235和Q450NQR1朝天面和朝地面的锈层截面形貌
Fig.5
Cross-section micro-morphologies of the rust layer on skyward (a-d) and field-ward (e-h) sides of Q235 and Q450NQR1
(a, e) Q235, 21 months (b, f) Q235, 34 months (c, g) Q450NQR1, 21 months (d, h) Q450NQR1, 34 months
对于耐候钢Q450NQR1来说,暴晒21个月时,两面锈层的厚度相近。暴晒34个月时,朝天面与朝地面的厚度差别较大,朝天面锈层的厚度约为朝地面的3.5倍,而朝地面锈层相对更致密。说明在南沙大气环境中暴晒34个月后,Q450NQR1的朝天面和朝地面截面的形貌差异比Q235大。从厚度上来看,耐候钢Q450NQR1的锈层厚度明显小于低碳钢Q235 (约1/3);从裂纹的分布上来看,Q235两面锈层中的裂纹明显多于Q450NQR1,更有利腐蚀介质的渗入。
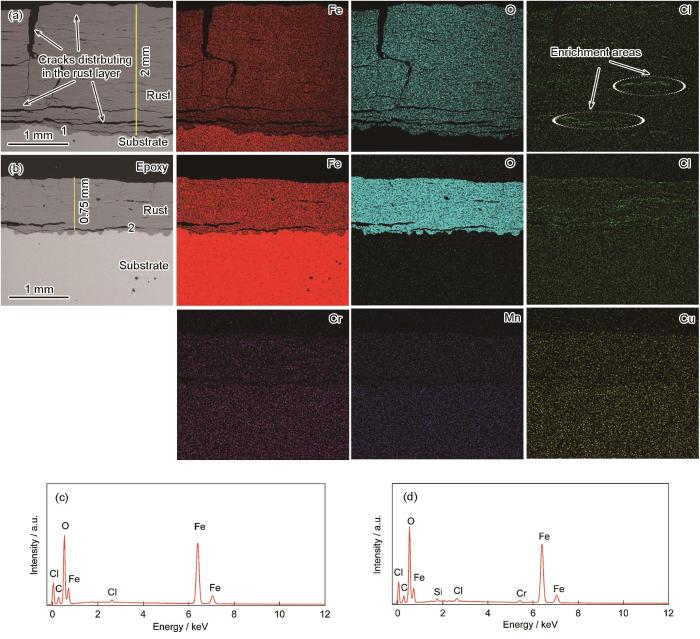
2种钢在暴晒34个月后朝天面锈层中的元素分布如图6所示。从图6a中可以看出,Q235表面锈层中Cl元素的分布相对集中在横向裂纹处,Q450NQR1表面锈层中的Cl元素相对分散,也多出现在裂纹处。从图6c中也可以看出,由于裂纹相对较窄,从而使Cl的聚积相对较少。此外,对于Q450NQR1来说,锈层中还零星分布着极少量的Cr、Mn和Cu元素(图6b)。对近基体处的元素进一步分析(图6d),发现Q450NQR1锈层中Cl元素的原子分数约为Q235中的1/2,还有微量的Cr元素。Cr作为提高耐候钢耐蚀性的主要元素之一,提高了锈层的致密度,细化腐蚀产物的颗粒[24,27]。已有研究[28~30]指出,合金元素Cr、Mn和Cu的加入能有效阻碍O2和腐蚀介质扩散到基体表面,降低锈层的导电性。因而,耐候钢Q450NQR1在南沙海洋大气环境中暴晒21个月后可形成保护性较好的锈层。由于34个月时,对低碳钢Q235和耐候钢Q450NQR1来说,朝天面和朝地面的锈层只是厚度不同,而结构相似,此处只对朝天面锈层的元素分布进行分析。
图6
图6
低碳钢Q235和耐候钢Q450NQR1暴晒34个月后朝天面的锈层截面形貌和元素分布
Fig.6
Cross-section morphologies and element distributions (EDS map) of rust layer on skyward side of Q235 (a) and Q450NQR1 (b) after 34 months' exposure, and EDS analyses of point 1 in Fig.6a (c) and point 2 in Fig.6b (d)
2.4 电化学分析
图7
图7
暴晒21和34个月后Q235和Q450NQR1的极化曲线
Fig.7
Potentiodynamic polarization curves of rusted Q235 (a) and Q450NQR1 (b) after exposure for 21 and 34 months (E—potential, i—current indensity)
利用Tafel外推法从极化曲线拟合得到的2种钢的腐蚀电位(Ecorr)和腐蚀电流密度(icorr)如表2所示。拟合参数中可以用icorr来评估腐蚀过程的动力学变化趋势。Q235在暴晒34个月时,朝天面的icorr从7.97 μA/cm2增大至16.98 μA/cm2,朝地面的icorr从98.54 μA/cm2增大至119.90 μA/cm2,说明暴晒21个月到34个月时,icorr有一定的增大趋势。对Q450NQR1来说,在暴晒34个月时,朝天面的icorr从7.11 μA/cm2减小至6.18 μA/cm2,而朝地面的icorr从80.06 μA/cm2增大至89.61 μA/cm2,耐候钢朝天面和朝地面的锈层随腐蚀时间的变化趋势略有不同,但这种变化趋势都很小。这也说明Q450NQR1在暴晒21个月到34个月时,锈层的保护性趋于稳定。对比2种钢朝天面和朝地面锈层的腐蚀情况发现,暴晒21个月和暴晒34个月,朝地面锈层的icorr都明显大于朝天面。这也说明朝地面锈层对腐蚀介质的阻碍作用更小,腐蚀更严重[33]。
表2 暴晒不同周期后的腐蚀电位(Ecorr)和腐蚀电流密度(icorr)
Table 2
| Steel | Ecorr / mV | icorr / (μA·cm-2) |
|---|---|---|
| Q235-21ms | -130.47 | 7.97 |
| Q235-21mf | -303.67 | 98.54 |
| Q235-34ms | -95.33 | 16.98 |
| Q235-34mf | -418.43 | 119.90 |
| Q450-21ms | -176.69 | 7.11 |
| Q450-21mf | -445.52 | 80.06 |
| Q450-34ms | -3.36 | 6.18 |
| Q450-34mf | -430.70 | 89.61 |
2.5 力学性能分析
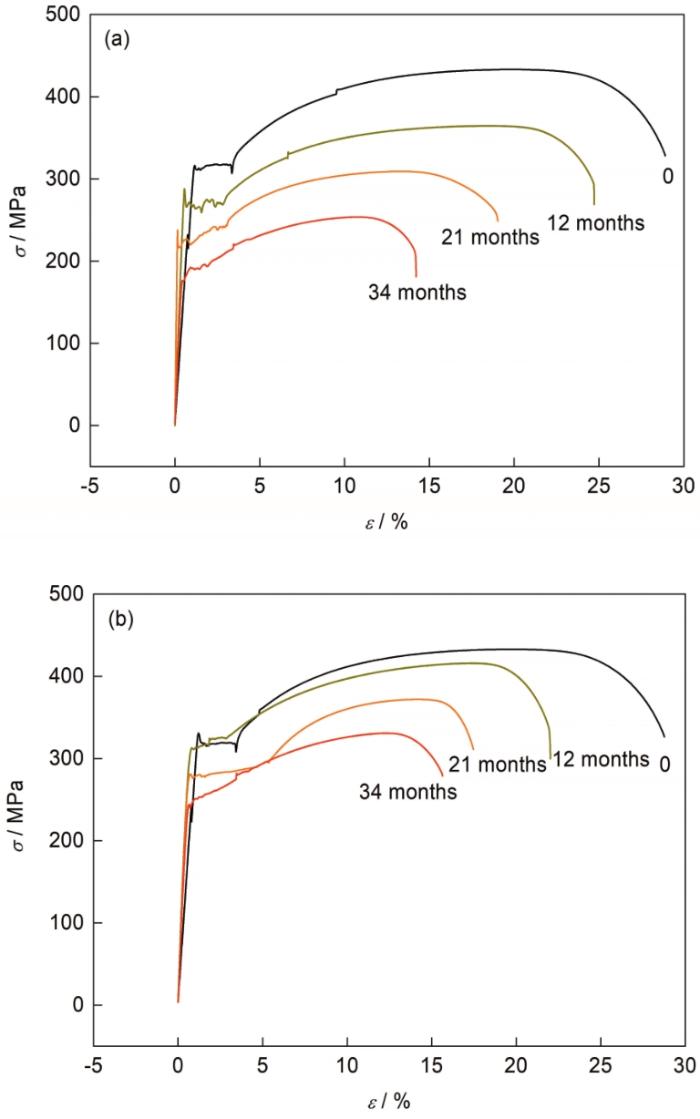
碳钢结构件在实际服役过程中或多或少都会受到一定的拉应力或压应力,因而力学性能随着碳钢锈蚀后的变化可为其评价腐蚀失效提供数据参考。图8为腐蚀前后2种钢的应力-应变曲线。从图中可知,腐蚀生锈后2种碳钢的抗拉强度和塑性均有较大的变化。抗拉强度随着暴晒时间的延长逐渐降低,塑性逐渐减小。这是由于随着暴晒时间的延长,锈层逐渐增加,钢基体的腐蚀深度逐渐增大,横截面积减小,而锈层的抗拉强度低于钢基体,从而使2种钢的抗拉强度下降。
图8
图8
Q235和Q450NQR1的应力-应变曲线
Fig.8
Stress (σ)-strain (ε) curves of Q235 (a) and Q450NQR1 (b) exposed for different periods
对比2种碳钢抗拉强度和延伸率的变化可知,低碳钢Q235的变化程度更大。结合截面形貌及失重数据结果,低碳钢Q235的锈层厚度比耐候钢Q450NQR1厚度大,基体的损失多,腐蚀后基体的横截面积小于Q450NQR1,从而造成了强度降低。因此,在南沙海洋大气环境中暴晒21个月后,耐候钢Q450NQR1的抗拉强度要明显高于低碳钢Q235。
2.6 腐蚀机理与讨论
从南沙海洋大气的环境参数可知,该地区相对湿度很高,极易在碳钢表面形成薄液膜,诱发电化学腐蚀过程的发生。即:Fe很快被氧化为Fe2+,Fe2+在润湿的大气中与O2反应生成FeOH+或Fe(OH)2,随后这些中间产物优先被氧化生成γ-FeOOH[27]。当空气中高浓度的Cl-被吸附到薄液膜中时,会生成一定量的β-FeOOH。此外,β-FeOOH还可由其他晶型的FeOOH经过重结晶转化而来[34]。而随着暴晒时间不断增加,锈层中的各产物会在一定条件下发生相互转化,如:锈层中的部分β-FeOOH和基体中的Fe发生反应形成Fe3O4;还原性较强的γ-FeOOH会有一部分转化为稳定的α-FeOOH,一部分会被继续还原为Fe3O4[33]。由于各产物的密度不同,它们在转化的过程中使锈层中局部体积发生变化,产生力的相互作用,引起了锈层的脱落。
由于受太阳直射和雨水冲刷等多方面的影响,造成了2种钢朝天面和朝地面腐蚀行为的差异。由于该地区地处赤道附近,太阳直射时间长和降水量大。晴天时,试样的朝天面的润湿时间会小于朝地面的润湿时间,使得朝地面电化学反应过程持续时间相对较长,腐蚀速率较快,这种情况在锈层相对较厚时更明显。雨天时,朝天面会受到雨水的冲刷作用,减少腐蚀性的沉积盐在样品表面的停留时间,从而使朝天面的腐蚀速率低于朝地面。
3 结论
(1) 碳钢在南沙海洋大气环境中腐蚀动力学过程分为2个阶段,第二阶段腐蚀速率较第一阶段小。耐候钢Q450NQR1在短期内就已体现出比低碳钢Q235更好的耐蚀性。
(2) 2种碳钢朝天面和朝地面锈层的主要相组成为γ-FeOOH、α-FeOOH、β-FeOOH和Fe3O4,各产物的相对含量随着暴晒时间的延长都有一定的差异。
(3) 暴晒21和34个月后,低碳钢Q235朝天面和朝地面的锈层均比耐候钢Q450NQR1的厚,且锈层中的裂纹更多,利于O2和Cl-向钢基体扩散,加速腐蚀过程。此外,2种钢的朝地面均比朝天面的腐蚀严重,这是由于朝地面的锈层极易脱落,使其对腐蚀介质的阻碍作用减弱。
(4) 随着暴晒时间的延长,碳钢Q235和耐候钢Q450NQR1表面锈层不断增加,使钢的抗拉强度逐渐降低。即在使用过程中随着锈层的增厚,Q235和Q450NQR1钢越容易失效,引发安全事故。
参考文献
Eight-year atmospheric corrosion exposure of steels in China
[J].
Atmospheric corrosion in Mauritius
[J].
Initial stages of atmospheric corrosion of steel in the Arabian Gulf
[J].
Corrosion of structural metals in atmospheres with different corrosivity at 8 years exposure in Sweden and Czechoslovakia
[J].
Atmospheric corrosion of mild steel. Part I—Rural and urban atmospheres
[J].
Atmospheric corrosion of mild steel. Part II—Marine atmospheres
[J].
Study of corrosion evolution of carbon steel exposed to an industrial atmosphere
[J].
Marine atmospheric corrosion of carbon steels
[J].
Initial atmospheric corrosion of carbon steel in industrial environment
[J].
Looking back on contributions in the field of atmospheric corrosion offered by the MICAT ibero-american testing network
[J].
The influence of sodium chloride on the atmospheric corrosion of steel
[J].
Atmospheric corrosion of steel in a humid tropical climate—Influence of pollution, humidity, temperature, solar radiation and rainfall
[J].
Characterization of atmospheric corrosion products on weathering steels
[J].
Effect of distance from sea on atmospheric corrosion rate
[J].
Long-term corrosion of cast irons and steel in marine and atmospheric environments
[J].
Characterisation of rust formed on carbon steel after exposure to open atmosphere in Qinghai salt lake region
[J].
Characterization of the rust formed on weathering steel exposed to Qinghai salt lake atmosphere
[J].
Analysis of the corrosion rust on weathering steel and carbon steel exposed in marine atmosphere for three years
[J].
海洋大气暴露3年的碳钢与耐候钢表面锈层分析
[J].
Atmospheric corrosion of low carbon steel in a coastal zone of Ecuador: Anomalous behavior of chloride deposition versus distance from the sea
[J].Atmospheric corrosion of low carbon steel exposed in a coastal tropical zone of Manabi, Ecuador, was determined. Specimens were exposed at six outdoor exposure sites located at different distances from the sea. The atmosphere is classified as coastal. Wind speed threshold for an increase in chloride deposition rate was determined. The behavior of chloride deposition rate versus distance from the sea is anomalous due to the presence of an estuary. Corrosion by weight loss was evaluated up to 1 year of exposure. Only a slight difference in atmospheric corrosion rate is noticed between wet and dry periods. Chloride deposition interaction with RH-temperature complex and with wind speed shows significant statistical influence on atmospheric corrosion of low carbon steel. Corrosivity category of the atmosphere high (C4) is the most predominant classification level in the zone. Prediction indicates corrosivity category high (C4) will remain up to 20 years of exposure. Different morphologies of corrosion products were identified by SEM. Lepidocrocite, goethite, magnetite, and akaganeite are the main crystalline phases determined by XRD. Protective ability index previously proposed for rust layers is not useful to apply in coastal sites.
Airborne chloride deposit and its effect on marine atmospheric corrosion of mild steel
[J].
Corrosion behavior of low-carbon steel and weathering steel in a coastal zone of the spratly islands: A tropical marine atmosphere
[J].
Corrosion of low carbon steel in atmospheric environments of different chloride content
[J].
Atmospheric corrosion of different steels in marine, rural and industrial environments
[J].
The long term growth of the protective rust layer formed on weathering steel by atmospheric corrosion during a quarter of a century
[J].
Influence of outer rust layers on corrosion of carbon steel and weathering steel during wet–dry cycles
[J].
The mechanism of atmospheric rusting and the protective amorphous rust on low alloy steel
[J].
In-depth distribution of rusts on a plain carbon steel and weathering steels exposed to coastal-industrial atmosphere for 17 years
[J].
Atmospheric corrosion of carbon steels and weathering steels in Taiwan
[J].
The influence of copper upon the atmospheric corrosion of iron
[J].
Electrochemical behavior of rust formed on carbon steel in a wet/dry environment containing chloride ions
[J].
On the protective nature of atmosph rust on low-alloy steel
[J].
Corrosion resistance and mechanical properties of low-alloy steels under atmospheric conditions
[J].
The mechanism of formation of iron oxide and oxyhydroxides in aqueous solutions at room temperature
[J].
The mechanisms of oxidation of ferrous hydroxychloride β-Fe2(OH)3Cl in aqueous solution: The formation of akaganeite vs goethite
[J].
Formation, fast oxidation and thermodynamic data of Fe(II) hydroxychlorides
[J].
Mechanisms of formation and structure of green rust one in aqueous corrosion of iron in the presence of chloride ions
[J].
The oxidation of ferrous hydroxide in chloride-containing aqueous media and pourbaix diagrams of green rust one
[J].
Electrochemical reduction of ferric corrosion products and evaluation of galvanic coupling with iron
[J].
Study of lepidocrocite γ-FeOOH electrochemical reduction in neutral and slightly alkaline solutions at 25oC
[J].
Atmospheric corrosion resistance of MnCuP weathering steel in simulated environments
[J].
Study on the rusting evolution and the performance of resisting to atmospheric corrosion for Mn-Cu steel
[J].The rust evolutions of the corrosion mass gain, the corrosion rate, the chemical composition, and the cross-section of the rust layer versus cycles of dry/wet alternate corrosion test were summarized on the basis of the study progress on the rust layer of Mn-Cu weathering steel. The validity of the cyclic dry/wet corrosion acceleration test simulating the atmospheric corrosion has also been discussed. Moreover, we have described the principle of synergistically improving the corrosion resistance of the rust layer resisting to both coastal atmospheric corrosion and industrial acidic rain atmospheric corrosion through adding alloying elements Cu, Mn and P. Meanwhile, the mechanism of P in the rust layer and the relationship of the ion-selectivity of Mn-Cu steel rust layer and its structure have been discussed.
Mn-Cu钢大气腐蚀锈层演化规律及其耐候性的研究
[J].根据Mn--Cu耐候钢大气腐蚀锈层的研究进展, 总结了低合金钢锈蚀的增重和腐蚀速率、锈层的组成和截面形貌等多个因素随干湿循环次数变化的演化规律, 阐述了所建立的室内干湿循环模拟大气腐蚀加速实验方法的合理性. 论述了以Cu, Mn和P为合金元素协同提高钢腐蚀锈层的耐海岸大气和工业酸雨大气腐蚀的原理, 介绍了Mn--Cu钢锈层的离子选择特性与结构关系的理论以及P在锈层中的作用机制.