随着可替代能源需求的不断增长以及环境污染问题的日益严重,直接甲醇燃料电池(DMFC)因其能量密度高、寿命长和环境友好等优点而极具应用潜力[1~3]。在DMFC中,常用金属Pt作为阳极催化剂。当前,该类燃料电池在应用过程中的主要缺点在于,甲醇氧化过程中产生的一些未完全氧化的CO中间体会吸附在Pt表面,占据Pt的活性位点,致使催化剂中毒,降低Pt的催化性能[3,4]。研究发现,在Pt中加入Fe[5]、Ni[6]、Co[7]等3d过渡金属元素和/或Ru[4]、Rh[8]、Ir[9]等贵金属元素作为助催化剂组成双/多金属催化剂,可在提高甲醇电催化活性的同时,促进COads氧化,提高催化剂抗中毒能力。在Pt-金属合金体系中,Pt-Ru系催化剂抗中毒能力最为突出[10],特别是PtRuFe[1]、PtRuNi[11]、PtRuMo[12]等三元催化剂被认为是极具应用前景的甲醇氧化催化剂。
目前,常用的DMFC阳极催化剂以负载型为主,其甲醇电催化活性仍有进一步提高的空间。从结构形态角度考虑,理想的催化剂应具有孔结构发达、电导率高、比表面积大等基本特征,如纳米花[2]、纳米线[9]以及纳米多孔结构[13]等。纳米多孔金属由纳米尺度的孔隙和金属韧带组成,具有独特的三维双连通多孔网状结构,具有良好的导电性和高的比表面积,被广泛应用于催化领域[13,14]。利用合金组元间的电极电位相差较大的特点,通过化学或电化学的作用腐蚀掉化学性质相对活泼的组元,剩余惰性组元通过表面扩散形成纳米多孔结构的脱合金化工艺,具有操作条件易于控制,成本低且易于实现工业化等优点,已经成为制备纳米多孔金属的常用手段[15,16]。理想的脱合金前驱体应满足单相区成分宽、合金组分间电势差大、非惰性金属组分含量高等特点[17]。固溶体合金是脱合金化工艺制备纳米多孔金属材料常用的前驱体[18]。除固溶体合金外,非晶合金没有晶界、位错等缺陷,化学成分均匀,成分范围宽,组元含量可调,且可形成非晶相的合金体系众多,组元种类丰富,也是一类适用于脱合金化制备纳米多孔金属的前驱体材料。通过对非晶合金脱合金化,已成功制备了Au[19]、Cu[20]、Pd[21]等多种纳米多孔单金属及Pt-Fe[22]、Cu-Ag[14]等纳米多孔双金属合金。但目前采用非晶合金前驱体脱合金化法制备三元及更多元纳米多孔金属的报道还较少。
本课题组前期通过对Fe-Pt-B非晶合金进行电化学脱合金化处理,获得了高甲醇电催化活性的纳米多孔PtFe合金[22]。为进一步提高PtFe合金的催化活性和抗中毒能力,本工作以Fe65Pt10B25 (原子分数,%,下同)合金为基础,加入适量Ru,首先制备Fe65Pt10-xRuxB25 (x=0~4)非晶合金带材,再将带材在H2SO4溶液中进行电化学脱合金化,成功制备出磁性纳米多孔(Pt, Ru)Fe合金,研究了Ru添加对纳米多孔合金的微结构、形貌和甲醇电催化性能的影响。结果表明,结合Ru、Fe对Pt的甲醇电催化活性和抗中毒能力的提升作用和纳米多孔金属比表面积大、活性点位多的特点,获得的PtRuFe三元纳米多孔合金具备优异的甲醇电催化性能。
1 实验方法
采用纯度高于99.95% (质量分数)的Fe、Pt、Ru和B原料按Fe65Pt10-xRuxB25 (x=0、2、4)名义成分进行称重配料。使用非自耗真空电弧炉在Ar气氛围下熔炼母合金锭,合金锭反复熔炼4次以保证成分均匀。通过单辊甩带装置制备宽约1.0 mm、厚约0.02 mm的带材。采用Interface 1000型电化学工作站,以急冷合金带材作为工作电极,以Ag/AgCl电极作为参比电极,以Pt片电极作为对电极,在0.1 mol/L的H2SO4溶液中进行恒电位脱合金化处理,x=0、2和4合金所用恒电位分别为-190、-170和-150 mV。采用D8 Focus 型X射线衍射仪(XRD,CuKα)和JEM-2010型透射电镜(TEM)表征脱合金前、后样品的微结构。采用JSM-7800F型扫描电镜(SEM)和附带的电子能谱仪(EDS)对脱合金后样品的形貌和成分进行观察分析。通过在0.5 mol/LH2SO4溶液中测得的循环伏安(CV)曲线表征脱合金后样品的电化学面积,扫描速率为50 mV/s,电化学活性表面积(ECSA)通过QH/210计算获得,其中QH为氢区电荷量,单位面积Pt表面吸附的电荷量以210 mC/cm2计算[1];通过在0.5 mol/L H2SO4+1.0 mol/L CH3OH混合溶液中的CV曲线评价脱合金后样品的甲醇电催化性能,扫描速率为20 mV/s。在相同条件下测试商用Pt/C催化剂(质量分数20%)的甲醇催化性能作为对比。使用ESCALAB 250Xi型X射线光电子能谱(XPS,AlKα)鉴定脱合金后样品表面元素组成和电子状态。利用Lakeshore-7400s型振动样品磁强计(VSM)在1432 kA/m最大外加磁场条件下测试合金的磁性能。
2 实验结果与讨论
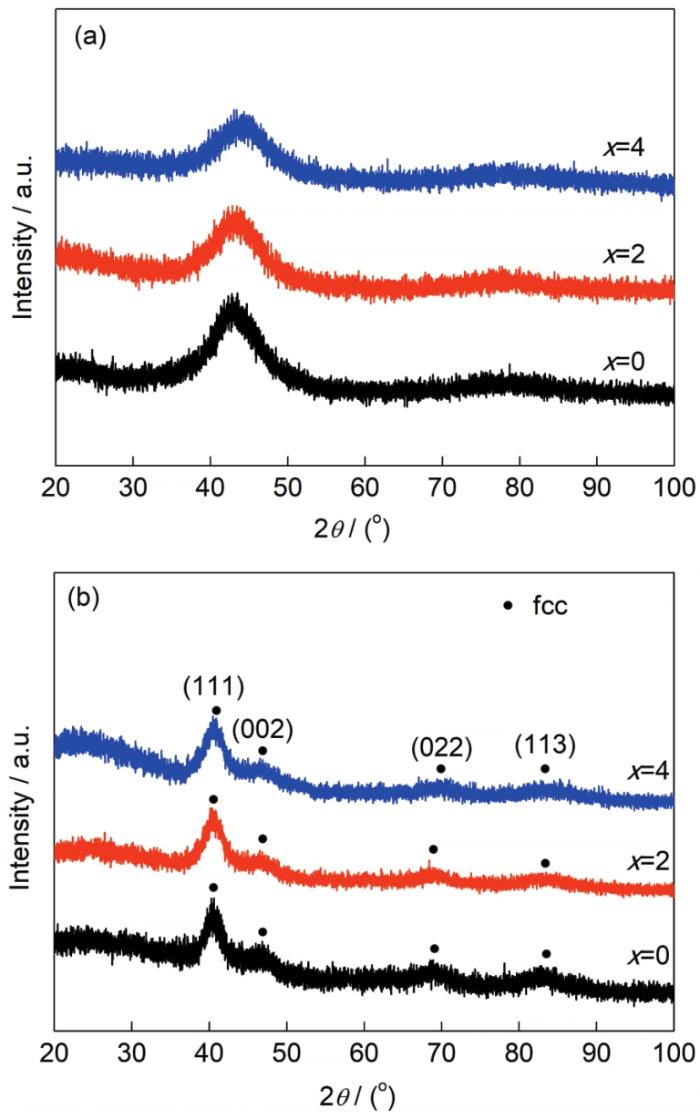
图1为Fe65Pt10-xRuxB25 (x=0~4)急冷合金带材及其脱合金后样品的XRD谱。从图1a可以看出,所有急冷合金带材的XRD谱均只有一个较宽的漫散射峰,无其它尖锐的晶体相衍射峰出现,表明合金均具有完全非晶态结构。如图1b所示,所有合金带材经脱合金后,XRD谱中的漫散峰均消失,在衍射角分别为41°、47°、69°和83°附近出现4个尖锐的衍射峰,依次对应于fcc结构Pt的(111)、(002)、(022)和(113)晶面。XRD谱中并未出现对应Ru或Fe相的衍射峰,说明合金脱合金后分别形成了PtFe (x=0)和PtRuFe (x=2、4)固溶体。与纯Pt (PDF No.04-0802)的衍射峰相比,3个合金的衍射峰均向大衍射角度方向偏移,这是因为原子半径相对较小的Fe和Ru固溶在fcc结构Pt中,晶格发生了一定程度的畸变,晶格常数减小[2]。
图1
图1
Fe65Pt10-xRuxB25 (x=0~4)急冷合金带材及其脱合金后样品的XRD谱
Fig.1
XRD spectra of melt-spun Fe65Pt10-xRuxB25 (x=0~4) ribbons before (a) and after (b) dealloying
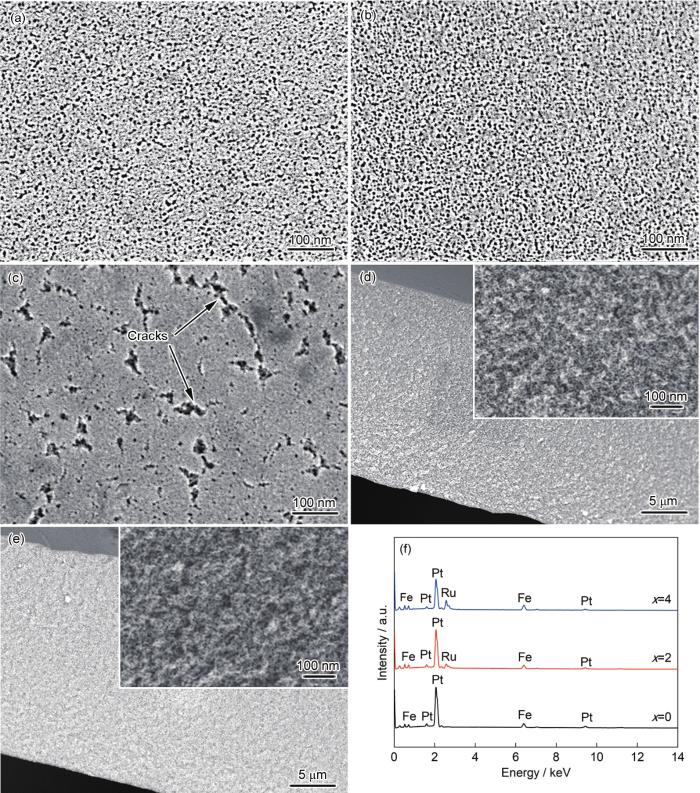
图2a~c为Fe65Pt10-xRuxB25 (x=0~4)非晶合金脱合金后样品表面形貌的SEM像。如图2a和b所示,x=0和2时,非晶合金经脱合金后,样品表面均具有由纳米尺度的孔隙和韧带组成的多孔结构。x=0时,合金的平均孔径(d)和韧带尺寸(l)分别为7和8 nm;当x=2时,合金多孔形貌变化不大,d和l分别为6和7 nm。当x=4时,非晶合金脱合金后表面形成的纳米孔数量较少,孔径分布均匀性较差,且出现多个孔聚集形成的裂纹,见图2c。统计除裂纹外的纳米孔,其d约为3 nm。图2d和e分别为x=2和x=4时合金的横截面形貌的SEM像,图2d和e中插图为局部放大像。可见,x=2时合金的整个横截面呈现孔隙、韧带双连续的三维纳米多孔合金典型的海绵状特征,表明脱合金化贯穿整个合金带材,样品内部同样形成了均匀的纳米多孔结构。x=4时,尽管合金表面形成的纳米多孔结构均匀性较差,但其横截面也呈现出海绵状纳米多孔结构,并未观察到多孔聚集而形成裂纹的情况。图2f为纳米多孔合金的EDS。可见,在2.05和9.44 keV处出现了Pt的特征能量峰;在2.56 keV处出现Ru的特征峰;在0.71和6.40 keV处出现Fe的特征峰,表明多孔合金由Pt、Ru和Fe元素组成。通过EDS分析得到的合金成分列在表1中。由表1可知,纳米多孔合金中贵金属(Pt或Pt+ Ru)和Fe的原子比均接近2∶1,远远高于原始合金中贵金属与Fe的比例,但Pt与Ru的比例与原始合金相当。尽管EDS无法准确测量轻元素B的含量,但因B在fcc结构FePt相中的固溶度极低[22],多孔(Pt, Ru)Fe合金中B含量十分有限。以上分析表明,在脱合金过程中,前驱体中的B和大部分Fe元素被选择性溶解,而Pt和Ru得以保留。表1还归纳了各纳米多孔合金的相结构和形貌特征参数(d、l)。
图2
图2
Fe65Pt10-xRuxB25非晶合金带材脱合金后的表面及横截面形貌的SEM像和EDS
Fig.2
Surface (a~c) and cross-sectional (d, e) SEM images and EDS (f) of Fe65Pt10-xRuxB25 amorphous alloy ribbons after dealloying (Insets in Figs.2d and e show the enlarged views)
(a) x=0 (b, d) x=2 (c, e) x=4
表1 Fe65Pt10-xRuxB25 (x=0~4)非晶合金带材脱合金后样品的相组成、平均孔径(d)和韧带尺寸(l)以及Pt、Ru和Fe的相对原子分数
Table 1
| x | Phase | d nm | l nm | Atomic fraction / % | ||
|---|---|---|---|---|---|---|
| Pt | Ru | Fe | ||||
| 0 | fcc-PtFe | 7 | 8 | 66.02 | - | 33.98 |
| 2 | fcc-PtRuFe | 6 | 7 | 52.02 | 13.23 | 34.75 |
| 4 | fcc-PtRuFe | 3 | - | 37.86 | 25.82 | 36.32 |
图3
图3
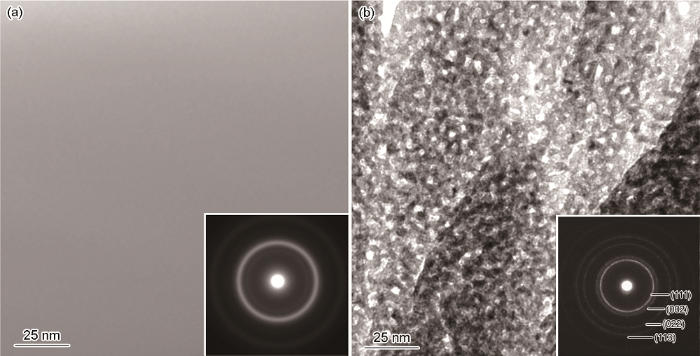
Fe65Pt8Ru2B25急冷合金带材及其脱合金后样品的TEM眀场像和选区电子衍射(SAED)花样
Fig.3
Bright-field TEM images and corresponding SAED patterns (insets) of melt-spun Fe65Pt8Ru2B25 ribbons before (a) and after (b) dealloying
Fe-Pt-Ru-B非晶合金经脱合金后形成的纳米多孔结构可基于表面扩散机制进行解释。Fe、Pt、Ru和B元素相对于标准氢电极的标准电位分别为-440 mV (Fe2+/Fe)、+1190 mV (Pt2+/Pt)、+455 mV (Ru2+/Ru)和-890 mV (B3+/B)[22]。在恒电位脱合金起始阶段,位于合金/溶液界面处相对活泼的B和Fe元素首先开始溶解,在界面位置形成空位;随着空位的增多,非晶结构失稳晶化,惰性元素Pt、Ru和剩余的Fe元素进行重组,生成更为稳定的fcc结构PtRuFe团簇/晶核;随后,Pt、Ru与Fe原子通过表面扩散,不断在界面迁移、聚集,形成纳米尺度韧带;内层B和部分Fe因Pt、Ru的迁移被暴露在固/液界面处,继续溶解形成新的空位,进而内部也形成三维连通的纳米多孔结构;最终,脱合金化贯穿整个带材样品,获得由单一fcc结构PtRuFe相构成的纳米多孔合金[21~23]。此外,非晶相所特有的无晶界缺陷、化学成分均匀的优势有助于获得孔隙和韧带均匀分布的纳米多孔结构[22,24]。
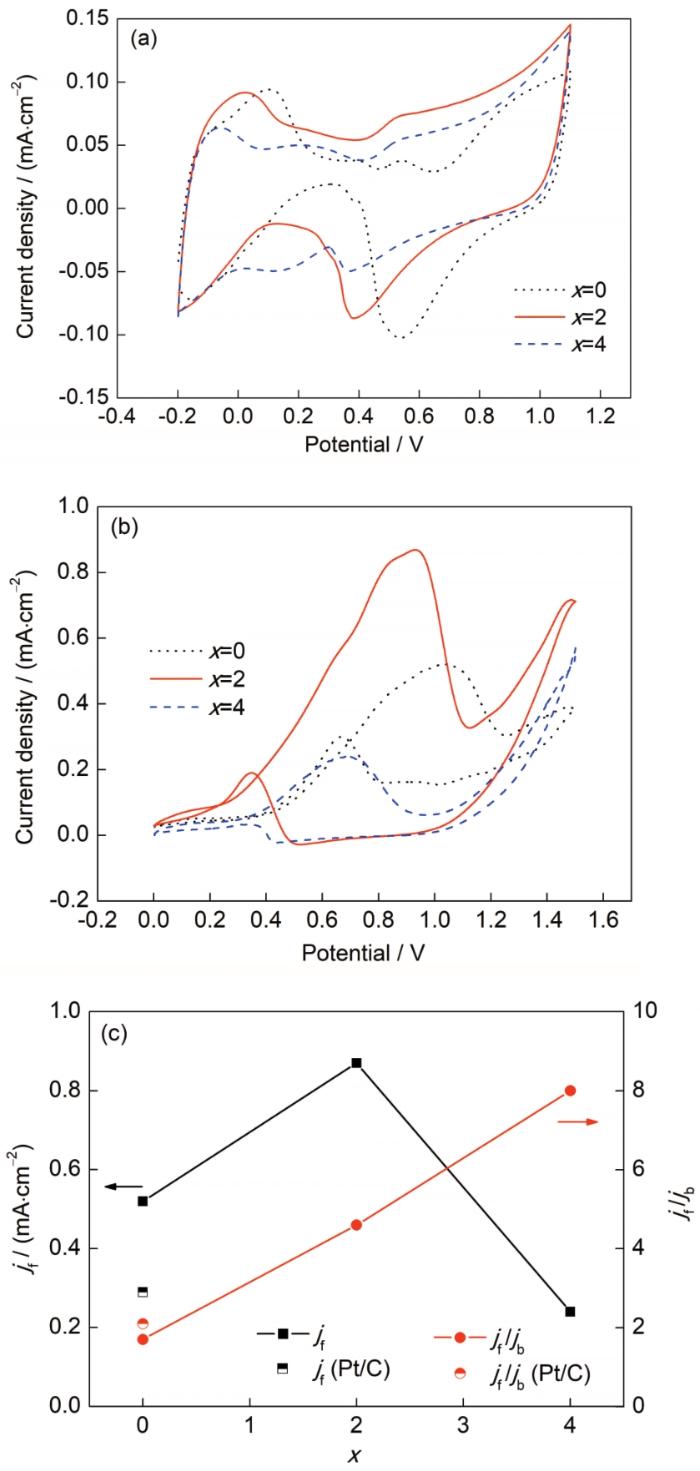
图4a为Fe65Pt10-xRuxB25 (x=0~4)非晶合金带材脱合金后形成的纳米多孔(Pt, Ru)Fe合金在0.5 mol/L H2SO4溶液中的CV曲线,其中电流密度按几何表面积归一化处理。可见,所有合金均具有相似的CV曲线特征,即包括氢吸附/脱附区、双电层区、氧化和还原区域。与纳米多孔PtFe合金相比,纳米多孔PtRuFe合金的还原峰随Ru含量增多而逐渐向负电位偏移,表明合金表面对含氧物种的吸附能力更强[5]。图4b为纳米多孔合金在0.5 mol/L H2SO4+1.0 mol/L CH3OH混合溶液中的CV曲线,其中电流密度根据图4a中CV曲线计算得到的电化学活性表面积归一化处理。可见,所有CV曲线均具有2个典型的甲醇氧化峰。其中,正向扫描的电流峰是由甲醇的脱氢和氧化引起的,而反向扫描中的电流峰是由未完全氧化的含C物质的氧化引起的[2]。通常采用氧化峰的电流密度和峰电位来评价催化剂的电催化活性。由图4b可知,随着Ru含量的增加,纳米多孔PtRuFe合金的正扫氧化峰电位(Ef)向负电位方向移动,表明其反应更易进行[25]。图4c为纳米多孔合金的正扫氧化峰电流密度(jf)和正、反扫氧化峰电流密度比值(jf/jb)与Ru含量的关系,商用Pt/C的数据也绘在图中作为对比。可见,x=2时合金的jf为0.87 mA/cm2,约为商用Pt/C (0.29 mA/cm2)的3倍,x=0时合金(0.52 mA/cm2)的1.7倍,表明其具有更高的甲醇电催化活性。但当x=4时,jf降低至0.24 mA/cm2,这是由于该合金的表面纳米多孔结构不均匀以及Pt含量降低造成了合金有效电化学面积减小,Pt活性位点减少。jf /jb通常用来评价催化剂的抗中毒能力[26]。由图4c可知,不含Ru元素的纳米多孔PtFe (x=0)和商用Pt/C的jf /jb较低,为1.7~2.1;随着Ru含量的增加,jf /jb显著增加,x=2和4时合金的jf /jb分别为4.6和8.0,分别是x=0时合金的2.7和4.7倍。这表明Ru的添加使得甲醇在正向电位扫描过程中被有效氧化,反向电位扫描过程中吸附的CO物种(COads)较少,即抗CO中毒能力逐渐增强。以上结果表明,在纳米多孔PtFe合金中加入适量Ru既可以提高甲醇电催化活性,有利于氧化反应的发生,又提高了催化剂抗中毒能力。
图4
图4
Fe65Pt10-xRuxB25 (x=0~4)非晶合金带材脱合金后的纳米多孔合金在0.5 mol/L H2SO4和0.5 mol/L H2SO4+1.0 mol/L CH3OH混合溶液中的循环伏安曲线,纳米多孔合金的甲醇氧化峰值电流密度(jf)及正、反扫氧化峰电流密度比值(jf /jb)与Ru含量的关系(商用Pt/C数据绘在图中作为对比)
Fig.4
CV curves of nanoporous alloys fabricated by dealloying Fe65Pt10-xRuxB25 (x=0~4) amorphous alloy ribbons in 0.5 mol/L H2SO4 (a) and 0.5 mol/L H2SO4+1.0 mol/L CH3OH solutions (b), and peak current density in the forward scan (jf) and ratio of peak current density in the forward scan to that in the backward scan (jf /jb) as a function of Ru content (The data of Pt/C are shown for comparison) (c)
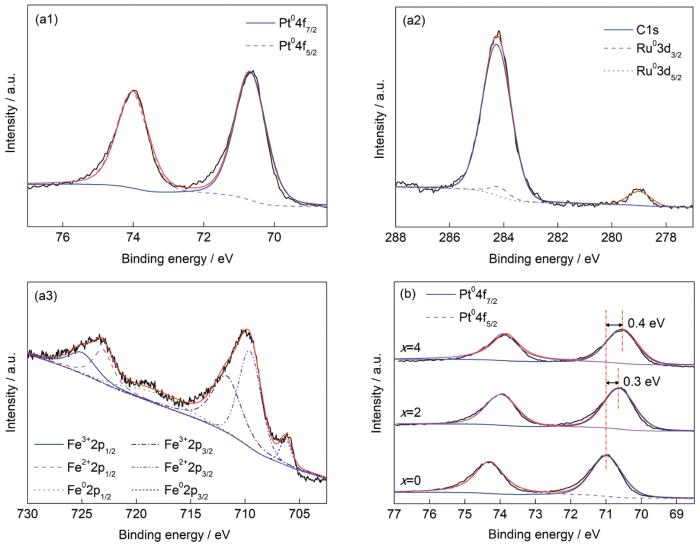
通过XPS对纳米多孔合金表面元素组成和电子价态进行了分析。宽谱扫描显示,PtRuFe纳米多孔合金仅含Pt、Ru和Fe 3种元素(忽略C、O等杂质元素),与EDS分析结果一致。图5a1~a3分别给出了x=2时非晶合金获得的纳米多孔合金中Pt4f、Ru3d和Fe2p的XPS谱。纳米多孔合金表面Pt和Ru主要以金属态存在,而Fe以金属态和氧化态并存。图5b是x=0~4时纳米多孔合金Pt4f的XPS谱。可见,Pt4f5/2和Pt4f7/2的结合能随x值的增加而逐渐降低,x=2和4时合金的对应峰位分别比x=0时合金负移0.3和0.4 eV。基于XPS分析结果,纳米多孔PtRuFe合金催化性能的改善源于双功能机制和电子效应。一方面,Pt在催化甲醇电氧化过程中,发生如下反应[27,28]:
图5
图5
Fe65Pt10-xRuxB25(x=0~4)非晶合金带材脱合金后的纳米多孔合金的XPS谱
Fig.5
XPS spectra of nanoporous alloys fabricated by dealloying Fe65Pt10-xRuxB25 (x=0~4) amorphous alloy ribbons(a1~a3) x=2: Pt4f, Ru3d and Fe2p, respectively (b) x=0~4: Pt4f
反应(1)释放的部分氧化中间体COads会吸附在Pt的活性中心,使Pt的催化效率降低。反应(2)生成的OHads可通过反应(3)去除部分COads。但对Pt而言,反应(2)需要在高电位(0.75 V)下发生,故Pt催化剂很容易因CO中毒而失效。在加入Ru后,在较低电位(0.35 V)下即可发生反应(4):
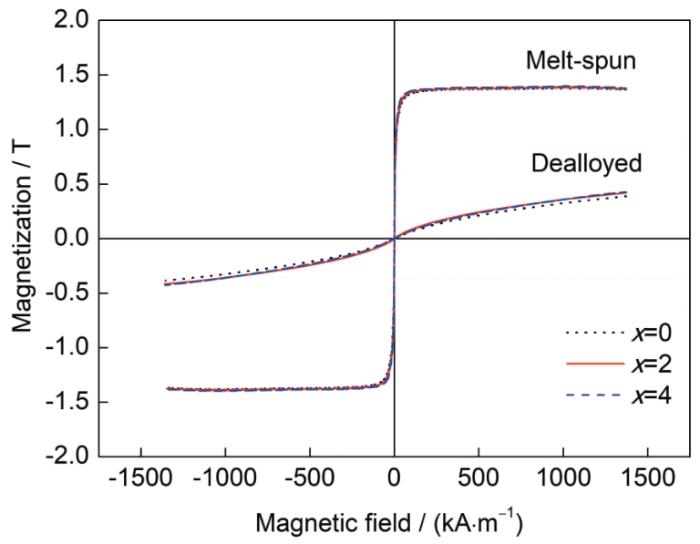
如图6所示,Fe65Pt10-xRuxB25 (x=0~4)急冷合金带材及脱合金后的纳米多孔合金均显示出典型软磁特征。非晶合金在脱合金前的饱和磁化强度(Ms)接近,为1.36~1.38 T。脱合金后,由于合金中部分Fe被溶解,Ms有所降低,x=2和4时合金的Ms分别为0.41和0.42 T,略高于PtFe合金(0.38 T)。结合EDS结果,这是由于含Ru纳米多孔合金中的Fe含量略高。在外磁场作用下,磁性纳米多孔合金催化剂可实现与反应物及产物的分离,在实际应用中易于回收再利用。
图6
图6
Fe65Pt10-xRuxB25 (x=0~4)急冷合金带材及脱合金后样品的磁滞回线
Fig.6
Hysteresis loops of Fe65Pt10-xRuxB25 (x=0~4) melt-spun alloy ribbons before and after dealloying
3 结论
以Fe65Pt10-xRuxB25 (x=0、2、4)非晶合金带材为前驱体,在0.1 mol/L H2SO4溶液中进行恒电位脱合金化,成功制备出由单一fcc结构(Pt, Ru)Fe相组成的磁性纳米多孔合金。x=0、2时前驱体脱合金后形成具有平均孔径为6~7 nm,韧带尺寸为7~8 nm的均匀双连通纳米多孔结构。随x由0增至4,纳米多孔合金在0.5 mol/L H2SO4+1.0 mol/L CH3OH混合溶液中的正扫氧化峰电位依次向负电位方向移动,正扫氧化峰电流密度先增加后降低,而正、反扫氧化峰电流密度比值逐渐增加。x=2时纳米多孔合金的正扫氧化峰电流密度和正、反扫氧化峰电流密度比值分别为0.87 mA/cm2和4.6,分别是x=0时合金的1.7和2.7倍,表明其具有比PtFe合金更高的甲醇电催化活性和抗CO中毒能力。纳米多孔PtRuFe合金还具有铁磁性,饱和磁化强度为0.41~0.42 T。优异的甲醇电催化性能及铁磁性使得纳米多孔PtRuFe合金在DMFC催化领域具有应用前景。
参考文献
Tailoring the composition of ultrathin, ternary alloy PtRuFe nanowires for the methanol oxidation reaction and formic acid oxidation reaction
[J].
Facile synthesis of binary PtRu nanoflowers for advanced electrocatalysts toward methanol oxidation
[J].
Highly active PtRuFe/C catalyst for methanol electro-oxidation
[J].
A comparative study of electrochemical methods on Pt-Ru DMFC anode catalysts: The effect of Ru addition
[J].
Synthesis of Pt and Pt-Fe nanoparticles supported on MWCNTs used as electrocatalysts in the methanol oxidation reaction
[J].
Preparation of PtNi nanoparticles for the electrocatalytic oxidation of methanol
[J].
Selective methodology for developing PtCo NPs and performance screening for energy efficient electro-catalysis in direct ethanol fuel cell
[J].
Facile synthesis of composition-tunable PtRh nanosponges for methanol oxidation reaction
[J].
PtIr alloy nanowire assembly on carbon cloth as advanced anode catalysts for methanol oxidation
[J].
High throughput experimental and theoretical predictive screening of materials—A comparative study of search strategies for new fuel cell anode catalysts
[J].
Electrodeposition of PtRu/MWCNTs and PtRuNi/MWCNTs and their performance in direct methanol fuel cells
[J].I_f/Ib (the forward anodic peak/the reverse anodic peak current) value and an appreciably improved resistance to carbon monoxide (CO) poisoning in methanol solution, so a beneficial effect on the oxygen adsorption in dilute sulphuric acid solution was observed. The high electrocatalytic activity and good stability of PtRuNi/MWCNTs was attributed to the synergetic effect of bifunctional catalysis, three dimension structure and oxygen functional groups which generated after electrochemical activation treatment on MWCNTs surface. The successful preparation of PtRu/MWCNTs and PtRuNi/MWCNTs nanocomposites opens a new path for efficient dispersion of promising electrocatalysts in DMFCs.]]>
电沉积制备PtRu/MWCNTs和PtRuNi/MWCNTs及其在直接甲醇燃料电池中的性能
[J]./多壁碳纳米管(PtRu/MWCNTs)和铂钌镍/多壁碳纳米管(PtRuNi/MWCNTs)金属纳米复合材料,用透射电子显微镜、能量色散光谱、X射线多晶衍射和X光电子能谱对其形貌、成分和结构进行表征和分析.采用循环伏安法和计时电流法研究PtRu/MWCNTs和PtRuNi/MWCNTs金属纳米复合材料对O还原(ORR)和甲醇氧化的电催化活性和稳定性.结果表明, PtRuNi/MWCNTs的ORR起始电位发生明显正移, 峰电流增高, 甲醇氧化峰电流高, 氧化峰电位负移,具有高抗CO中毒性能且稳定性好, 是良好的直接甲醇氧化燃料电池阳极催化剂候选材料.同时, PtRuNi/MWCNTs的高ORR和甲醇氧化电催化活性, 是双功能工作理论、特殊三维结构和MWCNTs表面经电化学活化处理生成含O官能团多种因素的共同作用结果.]]>
Composition optimization of PtRuM/C (M=Fe and Mo) catalysts for methanol electro-oxidation via combinatorial method
[J].
Nanoporous silver via electrochemical dealloying and its superior detection sensitivity to formaldehyde
[J].30Zn70 precursor alloys. The results reveal that applied potential or current has a significant influence on the composition, morphology and nanoporous structure of NPS. A bi-continuous NPS with an average pore size of 80 nm was obtained by electrochemical dealloying in 0.1 mol/L HCl solution at a constant current density of 2.5 mA/cm2 for 6000 s. The cyclic voltammetry experiment results showed that NPS has a superior formaldehyde catalysis and detection abilities in 0.5 mol/L KOH solution due to the optimal combination of nanopores and Ag ligaments in nanoporous structure. The higher formaldehyde catalysis and detection abilities were exhibited at the NPS with smaller nanopores. The detection sensitivity of formaldehyde in NPS with the pore size of 80 nm was 0.22 mA·cm-2·(mmol·L-1)-1 in the concentration range of 10~100 mmol/L, and the peak current density was 25.0 mA/cm2 in 0.5 mol/L KOH solution with 100 mmol/L HCHO.]]>
电化学脱合金制备纳米多孔Ag及其甲醛检测性能
[J].30Zn70合金为原料,通过调控脱合金电势及电流的2种电化学脱合金方法制备纳米多孔Ag材料。结果表明,脱合金电位或电流对纳米多孔Ag的成分、结构及孔径尺寸有重要影响。通过在2.5 mA/cm2电流密度下脱合金处理6000 s后可获得孔径约为80 nm的双连续纳米多孔Ag结构。循环伏安实验结果表明,纳米多孔Ag在0.5 mol/L的KOH溶液中对甲醛有良好的催化和检测性能,归因于纳米多孔结构中较优的纳米多孔孔径和Ag韧带的尺寸匹配。具有更小尺寸孔径的纳米多孔Ag有着更高的甲醛催化和检测性能。孔径约为80 nm的纳米多孔Ag在10~100 mmol/L浓度范围内的甲醛检测灵敏度达到0.22 mA·cm-2·(mmol·L-1)-1;在含有100 mmol/L HCHO的0.5 mol/L KOH溶液中的催化峰值电流密度达到25.0 mA/cm2。]]>
Formation of nanoporous Cu-Ag by dealloying Mg-Cu-Y-Ag amorphous alloys and its electrocatalyst oxidation property
[J].
Nanoporous Pt-Co alloy nanowires: Fabrication, characterization, and electrocatalytic properties
[J].Nanoporous Pt-Co alloy nanowires were synthesized by electrodeposition of Co-rich Pt(1)Co(99) alloy into anodic aluminum oxide (AAO) membranes, followed by a dealloying treatment in a mild acidic medium. These nanowires consist of porous skeletons with tiny pores of 1-5 nm and crystalline ligaments of 2-8 nm. Morphological and compositional evolutions of the porous Pt-Co nanowires upon dealloying were investigated, and their formation mechanism is discussed. The nanoporous Pt-Co alloy nanowires are found to exhibit distinctly enhanced electrocatalytic activities toward methanol oxidation as compared to the current state-of-the-art Pt/C and PtCo/C catalysts, thus showing substantial promise as efficient anode electrocatalysts in direct methanol fuel cells.
Evolution of nanoporosity in dealloying
[J].Dealloying is a common corrosion process during which an alloy is 'parted' by the selective dissolution of the most electrochemically active of its elements. This process results in the formation of a nanoporous sponge composed almost entirely of the more noble alloy constituents. Although considerable attention has been devoted to the morphological aspects of the dealloying process, its underlying physical mechanism has remained unclear. Here we propose a continuum model that is fully consistent with experiments and theoretical simulations of alloy dissolution, and demonstrate that nanoporosity in metals is due to an intrinsic dynamical pattern formation process. That is, pores form because the more noble atoms are chemically driven to aggregate into two-dimensional clusters by a phase separation process (spinodal decomposition) at the solid-electrolyte interface, and the surface area continuously increases owing to etching. Together, these processes evolve porosity with a characteristic length scale predicted by our continuum model. We expect that chemically tailored nanoporous gold made by dealloying Ag-Au should be suitable for sensor applications, particularly in a biomaterials context.
An atomistic description of dealloying: Porosity evolution, the critical potential, and rate-limiting behavior
[J].
Characterization and mechanical behavior of nanoporous gold
[J].
Nanoporous gold by dealloying of an amorphous precursor
[J].
Fabrication of novel nanoporous copper powder catalyst by dealloying of ZrCuNiAl amorphous powders for the application of wastewater treatments
[J].The nanoporous copper (Cu) powders (NPCPs) co-existing with Cu2O was fabricated by dealloying of atomized Zr-Cu-Ni-Al amorphous powders. The as-fabricated NPCPs, with an inner core and outer shell, showed a large specific surface area of 7.52m(2)g(-1) and exhibited significantly superior degradation ability in the presence of hydrogen peroxide (H2O2) for the complete elimination of methyl orange (MO) under both acidic and neutral environments. The enhanced catalytic decomposition properties of H2O2 by NPCPs were mainly attributed to the large specific surface area and three-dimensional continuous nanoporous structure with unique atomic steps on the ligament surfaces. The mechanistic investigations revealed that Cu2O/H2O2 reactions in acidic environment and Cu(0)/H2O2 reactions in neutral environment, respectively, were responsible for the high degradability of azo dyes, indicating that NPCPs/H2O2 could be a good Fenton-like reagent in application of wastewater treatments.
Formation of ultrafine spongy nanoporous metals (Ni, Cu, Pd, Ag and Au) by dealloying metallic glasses in acids with capping effect
[J].
Fabrication and electrocatalytic properties of ferromagnetic nanoporous PtFe by dealloying an amorphous Fe60Pt10B30 alloy
[J].
Nucleation and growth of nanoporous copper ligaments during electrochemical dealloying of Mg-based metallic glasses
[J].
Elaboration of nanoporous copper by modifying surface diffusivity by the minor addition of gold
[J].
Dealloying to nanoporous Au/Pt alloys and their structure sensitive electrocatalytic properties
[J].A simple and general dealloying method is employed to fabricate nanoporous Au/Pt alloys with pre-determined alloy compositions. Structural characterization by electron microscopes demonstrates that selective etching of Cu from Au/Pt/Cu alloy precursors results in the formation of three-dimensional bicontinuous porous network structures with uniform pores and ligaments less than 10 nm. X-Ray photoelectron spectroscopy and X-ray diffraction demonstrate that nanoporous Au/Pt alloys have a single-phase cubic structure with relatively uniform compositions across the samples. These high surface area alloy nanostructures show much enhanced specific activity and distinct surface reactivity toward the electrooxidation of some small organic molecules, such as methanol and formic acid, as the Au content varies within the structure, thus holding great potential for use in clean energy and environmental applications.
Ternary PtRuFe nanoparticles supported N-doped graphene as an efficient bifunctional catalyst for methanol oxidation and oxygen reduction reactions
[J].
Particle size effect of carbon-supported platinum catalysts for the electrooxidation of methanol
[J].
Recent development of methanol electrooxidation catalysts for direct methanol fuel cell
[J].
Surface Ru enriched structurally ordered intermetallic PtFe@PtRuFe core-shell nanostructure boosts methanol oxidation reaction catalysis
[J].
Ultrathin branched PtFe and PtRuFe nanodendrites with enhanced electrocatalytic activity
[J].
PtRu alloy nanoparticles supported on nanoporous gold as an efficient anode catalyst for direct methanol fuel cell
[J].