1 实验方法
1.1 Rb纳米溶胶的制备
Rb纳米颗粒的制备采用固/液转变+超声分散的方法,如图1所示。首先,在N2手套箱中(O2和H2O浓度分别小于10 × 10-6和1 × 10-6),切20 g钾块置于200 mL甲苯溶液中,于90℃加热搅拌1.5 h直至钾块的新鲜暴露面不再氧化变色,说明甲苯中的O2和H2O已经被完全除去(步骤I);接着,将装有金属Rb的安瓿瓶在50℃的加热台上加热至金属Rb熔化,迅速切割开安瓿瓶,取10 μL液态金属Rb和10 mL已去除O2和H2O的甲苯共同放入透明样品瓶中,盖好瓶盖,再利用石蜡密封住瓶口(步骤II)。
图1
图1
固/液转变+超声分散法制备Rb纳米溶胶的示意图
Fig.1
Schematic of the rubidium sol preparation via solid/liquid transformation and ultrasonic dispersion
然后,将密封好的样品瓶从手套箱中取出并放置在功率可调的KQ-400DE数控超声波清洗器水池中,于80℃水浴加热。样品瓶中的Rb再次熔化成液态并沉在瓶中的底部,以40 kHz的频率和400 W的功率超声5 min,在此过程中,可以观察到Rb液滴逐渐消失,溶液的颜色从无色变成蓝灰色(步骤III)。最后,将样品瓶从超声池中取出冷却,即获得了甲苯分散的Rb纳米溶胶(步骤IV)。类似地,通过调节超声功率至320 W和240 W,可获得系列胶体溶液。
1.2 表征
在UV-3600紫外-可见-近红外分光光谱仪上进行Rb纳米溶胶的吸收光谱测试,并将纯甲苯的光吸收谱线作为基线。在N2手套箱中,使用移液枪将制备的胶体溶液滴在深度为2 mm、直径为15 mm的玻璃槽内,加热使甲苯溶剂挥发后残留物质形成粉末,用聚酰亚胺胶带密封住粉末样品以隔绝O2和H2O,采用X'Pert X射线衍射仪(XRD)进行物相分析,所使用的X射线为CuKα。将胶体溶液滴在铜网上,干燥后采用JEM-2010透射电子显微镜(TEM)对样品进行微结构的表征。使用TEM上配备的IE250X-Max50能谱仪(EDS)进行元素分析。
1.3 燃烧特性的评估
将陶瓷舟放置于加热台上加热至一定温度(温度由红外测温计测量),取300 μL的金属Rb纳米溶胶滴入陶瓷舟内,利用摄像机记录胶体溶液的着火、燃烧过程。通过Adobe Premiere软件慢速播放并截取特定时间或状态下的照片来研究相关的燃烧行为。
2 实验结果与讨论
图2
图2
所制备的Rb溶胶的光学吸收谱、溶胶装在样品瓶中光学照片及稀释10倍后的Tyndall效应
Fig.2
Optical absorption spectrum of the as-prepared sol solution (Inset I shows the optical photograph of the as-prepared sol in sample bottle; inset II shows the Tyndall effect of the sol after it was diluted to one-tenth of the original)
通过观察胶体溶液的颜色(纳米Rb的团聚、氧化均会造成颜色变化),发现在室温下,这样的储存方式保存半个月后,溶胶颜色无明显变化(尽管底部似有沉淀物,但可通过超声重新分散),表明通过这种方法制备、保存的溶胶具有较好的稳定性。若将装有Rb溶胶的蜡封样品瓶再通过真空袋包装,并放置在-18℃的冷冻环境中,则可以存放3个月以上。这说明一个良好的存储方式需要隔绝O2、H2O和保持低的保存温度。
2.1 形貌与结构
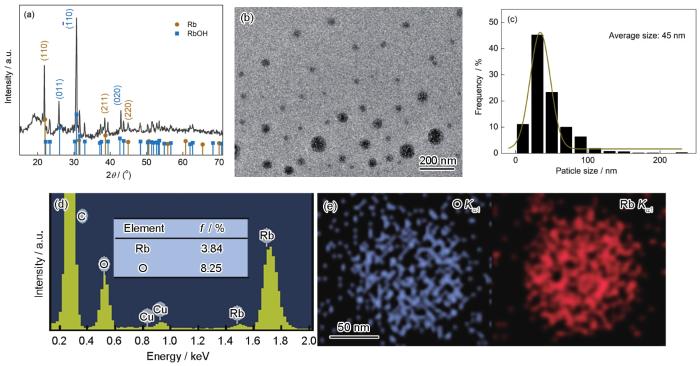
图3
图3
所制备的Rb溶胶中产物的表征
Fig.3
Characterization of the products
(a) XRD spectrum (b) TEM image (c) size distribution
(d) EDS result (f—atomic fraction) (e) element mapping of an isolated nanoparticles (NPs)
2.2 尺寸的可控性
图4
图4
不同超声功率下获得的溶胶的光学吸收谱、溶胶中产物的TEM像及粒径统计
Fig.4
Optical absorption spectra under different ultrasonic powers (Insets show the photos of the corresponding sols) (a), TEM images (b, d) and size distributions (c, e) of sols obtained with ultrasonic powers of 320 W (b, c) and 240 W (d, e)
2.3 Rb纳米溶胶的形成
图5
图5
Rb纳米溶胶的形成过程示意图
Fig.5
Schematics of the formation of rubidium sol
(a) ultrasonic cavitation effect
(b) schematic of the formation of rubidium NPs in toluene (I: cavitation bubble collapse induced mutual sputtering of rubidium and toluene at their interface, which produces relatively large liquid rubidium particles; II: smaller rubidium droplets produced via repeated cavitation bubble collapse; III: rubidium droplets reach critical size to form sol)
在本实验的体系中,在超声波作用下,许多小气泡会在甲苯和Rb液滴中形成、长大进而崩灭。气泡崩灭瞬间将产生局部的高压、高热微流,使金属Rb液滴与甲苯溶液在界面处互相溅射,分离出许多较大的金属Rb液滴,并在表面张力的作用下转变为近球形,如图5b中的步骤I所示。随着超声空化过程不断进行,这些分离出来的较大的Rb液滴也会反复在空化效应的作用下形成更小的Rb液滴,并分散在甲苯中,如图5b中的步骤II所示。当小的Rb液滴的表面张力与超声造成的应力达到平衡时,液滴将维持一个稳定的尺寸[25,26]。冷却后,Rb液滴凝固为固态颗粒,形成甲苯分散的金属Rb纳米颗粒溶胶,如图5b中的步骤III所示。由于在固定频率的情况下,一定范围内随着超声波功率增大,空化作用增强[27],所以,最终的金属Rb纳米颗粒尺寸就越小(如图4所示)。
3 点火特性
图6
图6
将300 μL的甲苯/金属Rb纳米溶胶置于120℃的陶瓷舟中不同时间的点火特性
Fig.6
Photos at intervals of 0 s (a), 1.06 s (b), 1.25 s (c), 2.05 s (d), 4.19 s (e), and 4.75 s (f) after 300 μL sol was placed in the ceramic boat at 120oC
图7
图8
图8
在120℃陶瓷舟中的溶胶液膜的形态演变及引燃示意图
Fig.8
Evolution of liquid film morphology in the ceramic boat at 120oC (a) and schematic of the ignition principle (b)
由于N2手套箱的密闭性有限,这种方法制备的纳米Rb颗粒的表面将会不可避免地被氧化,而氧化生成的Rb的氧化物或RbOH的存在会减慢纳米Rb的氧化速率、降低产生的热量,从而会降低Rb颗粒的点火性能。如果能够完全隔绝H2O和O2,将能够制备出点火性能更好的Rb纳米颗粒。
4 结论
依据Rb的低熔点特点和超声的乳化效应,提出并建立了固/液转变+超声分散的Rb的纳米化策略,即通过将熔化的液态Rb置于惰性的甲苯介质中进行超声分散、进而冷却凝固实现了Rb的纳米化,获得了甲苯分散的金属Rb纳米溶胶。Rb纳米颗粒尺寸可通过调节超声功率进行控制。该方法具有良好的可控性,既便捷又高效,适合于批量制备Rb纳米溶胶。同时,这种方法也可用来制备其他低熔点活泼金属的纳米颗粒。由于金属Rb纳米颗粒易于氧化从而形成局部的高温点,它可以对有机物(甲苯等)进行快速的引燃。
参考文献
Properties and research progress of rubidium and its compounds
[J].
铷及含铷材料的性能与应用研究进展
[J].
The design of high precision time reference measurement system based on rubidium disciplined crystal oscillator and double TDC GP2
[J].
基于铷原子钟和双TDC-GP2的高精度时间基准测量系统的设计
[J].
Influences of alkali metal Rb doped Mn as catalyst on catalytic combustion activity of soot particle
[J].
碱金属Rb掺锰对碳烟颗粒催化燃烧活性的影响
[J].
Effect of extracellular potassium on the activity of distal renal tubule in mice
[J].
细胞外钾对小鼠远端肾小管离子通道活性的影响
[J].
Properties of nanometer materials and its preparing methods
[J].
纳米材料的性质及其制备方法
[J].
Efficient raman enhancement in molybdenum disulfide by tuning the interlayer spacing
[J].
Preparation and application of Fe3O4 nanomaterials
[J].
四氧化三铁纳米材料的制备与应用
[J].近年来磁性Fe<sub>3</sub>O<sub>4</sub>纳米材料因其独特的物理化学性质如量子尺寸效应、表面界面效应、电学特性以及磁学特性等,而引起了广泛的研究,并在诸多领域(如环境、能源)具有潜在应用前景。本文总结了近年来国内外制备Fe<sub>3</sub>O<sub>4</sub>的一些方法,主要包括:沉淀法、热分解法、水热法、微乳液法以及溶胶-凝胶法,同时对各种制备方法的优缺点进行了比较。在应用方面,着眼于Fe<sub>3</sub>O<sub>4</sub>良好的磁响应性,综述了Fe<sub>3</sub>O<sub>4</sub>纳米材料及其复合物作为吸附剂用于去除废水中的金属离子以及有机污染物;系统总结了Fe<sub>3</sub>O<sub>4</sub>在催化中的应用,包括其本身作为催化剂和作为催化剂活性组分(如贵金属纳米粒子、金属氧化物半导体纳米光催化剂、金属有机化合物等)的载体两个方面。另外,本文还介绍了Fe<sub>3</sub>O<sub>4</sub>纳米材料在能源存储(锂离子电池和超级电容器)以及生物医药(肿瘤诊疗、固定化酶和免疫分析)等方面的应用。最后,针对目前Fe<sub>3</sub>O<sub>4</sub>纳米材料在制备中存在的一些问题进行探讨并对今后的研究方向进行了展望。
Preparation, doping modulation and field emission properties of square-shaped GaN nanowires
[J].
四方结构GaN纳米线制备、掺杂调控及其场发射性能研究
[J].
Design principle of all-inorganic halide perovskite-related nanocrystals
[J].
On the drastically improved performance of Fe-doped LiMn2O4 nanoparticles prepared by a facile solution-gelation route
[J].
Creating carbon-oxygen bonds over TiO2 nanofibers for synergistic benefits of visible-light response and charge separation toward photocatalysis
[J].
Characterization of alkali-metal sols in diethyl ether. Visible extinction and surface-enhanced Raman spectra
[J].
Characterization and surface-enhanced Raman spectroscopy of alkali metal sols
[J]. J.
A new method of production of colloid solutions
[J].
Concerning the manufacture of colloids from the condensation method of molecular rays
[J].
Ultrasonic nano-emulsification—A review
[J].The emulsions with nano-sized dispersed phase is called nanoemulsions having a wide variety of applications ranging from food, dairy, pharmaceutics to paint and oil industries. As one of the high energy consumer methods, ultrasonic emulsification (UE) are being utilized in many processes providing unique benefits and advantages. In the present review, ultrasonic nano-emulsification is critically reviewed and assessed by focusing on the main parameters such pre-emulsion processes, multi-frequency or multi-step irradiations and also surfactant-free parameters. Furthermore, categorizing aposematic data of experimental researches such as frequency, irradiation power and time, oil phase and surfactant concentration and also droplet size and stability duration are analyzed and conceded in tables being beneficial to indicate uncovered fields. It is believed that the UE with optimized parameters and stimulated conditions is a developing method with various advantages.Copyright © 2018 Elsevier B.V. All rights reserved.
Synthesis of sub-micronic and nanometric PMMA particles via emulsion polymerization assisted by ultrasound: Process flow sheet and characterization
[J].PMMA particle synthesis was performed from MMA (methyl methacrylate) and water mixtures, exposed to different ultrasonic systems and frequencies. The sonication sequence was 20kHz→580kHz→858kHz→1138kHz, and the solution was sampled after each irradiation step for polymerization. Effects of sonication parameters (time, power), polymerization method (thermo-initiated or photo-initiated), use of small amounts of surfactant (Triton X-100™ or Tween® 20) and initial MMA quantity were investigated on particle size and synthesis yields. Particle size and size distribution were measured by DLS (Dynamic Light Scattering), and confirmed via SEM (Scanning Electron Microscopy) images. Synthesis yield was calculated using the dry weight method. Particle composition was estimated using FTIR (Fourier Transform Infra-Red) spectroscopy. PMMA (polymethylmethacrylate) monodispersed particles were successfully synthesized, with a possibility of control in the 78-310nm size range. These sized-controlled particles were synthesized with a 7.5-85% synthesis yield (corresponding to 7.5-40g/L particle solid content), depending on operational parameters. Furthermore, a trade-off between particle size and synthesis yield can be proposed: 20kHz→10min waiting time→580kHz→858kHz leading to 90nm particles diameter with 72% yield in less than 40min for the whole sequence. Thus, the synthesis under ultrasound can be found easy to implement and time efficient, ensuring the success of the scale-up approach and opening up industrial applications for this type of polymeric particles.Copyright © 2017 Elsevier B.V. All rights reserved.
Optimization and characterization of ultrasound assisted preparation of curcumin-loaded solid lipid nanoparticles: Application of central composite design, thermal analysis and X-ray diffraction techniques
[J].This study is devoted to preparation of novel solid lipid nanoparticles (SLNs) for the encapsulation of curcumin which is produced by micro-emulsion and ultrasonication using stearic acid and tripalmitin as solid lipids, tween80 and span80 as surfactants. The relation between particle size and entrapment efficiency of the produced SLNs was operated by central composite design (CCD) under response likes surface method (RSM). The variables including the ratio of lipids (X), the ratio of surfactants (X), drug/lipid ratio (X), time of sonication (X) and time of homogenization (X). Particle size and entrapment efficiency of the loaded curcumin was justified according to the minimum particle size and maximum entrapment efficiency. The curcumin loaded SLNs presented fairly spherical shape with the mean diameter and entrapment efficiency of 112.0±2.6nm and 98.7±0.3%, respectively. The optimized SLNs were characterized by X-ray diffraction analysis (XRD), differential scanning calorimetry (DSC), photon correlation spectroscopy (PCS) and field emission scanning electron microscopy (FESEM). The drug release profile of the optimal formulated material was examined in aqueous media and almost 30% of the curcumin loaded in SLNs was gradually released during 48h, which reveals efficient prolonged release of the drug.Copyright © 2017 Elsevier B.V. All rights reserved.
Effects on SPR of Au/TiO2 by preparation methods
[J].
Au/TiO2的制备方法对其SPR的影响
[J].
Preparation and characterization of nano-gold particles by seeding method
[J].
纳米金颗粒的晶种法制备及表征
[J].
Applications of ultrasound in preparation of nanosized materials
[J].
超声技术在纳米材料制备中的应用
[J].
Which parameters affect biofilm removal with acoustic cavitation? A review
[J].
Effect rules and function mechanism of ultrasonic field on the solidification of high strength aluminum alloy
[D].
超声场对高强铝合金凝固过程的影响规律与作用机理研究
[D].
Mechanism for ultrasonic driving filling of liquid solder and acoustic cavitation
[D].
液态钎料超声驱动填缝机理及声空化作用研究
[D].
Review on applicable breakup/coalescence models in turbulent liquid-liquid flows
[J].
Influence of preparation method on size distribution of the dispersed phase of primary emulsions
[J].