为了确定连铸出现一系列问题的根源,研究者对不同体系中包晶相变的行为和机理进行了广泛的研究[5~10]。Boettinger等[5]将包晶相变过程分为包晶反应(L+δ→γ,其中L代表液相)和包晶转变(δ→γ或L→γ) 2个环节。包晶反应是包晶相γ在L/δ界面形核和生长的过程,当包晶相γ将液相L与初生相δ完全隔开时,包晶反应结束。包晶转变是指包晶相γ分别向初生相δ和液相L内生长的过程。Shibata等[6]发现,包晶反应中包晶相γ的生长伴随着初生相δ的溶解,在δ/γ/L三相交界点出现了明显的凹陷。Griesser等[7]指出,当温度保持恒定时,由于δ/γ/L三相表面张力保持平衡,包晶相γ生长需要通过溶质原子在液相中的局部短程扩散实现初生相δ的溶解和局部重凝来维持;而溶质的扩散驱动力来自于L/δ与L/γ界面处的液相浓度差。Chuang等[8]和Fredriksson 等[9]研究碳钢包晶转变时发现,包晶转变速率受控于液相中的溶质原子穿过γ相到达δ相的扩散速率,并且γ/δ界面的移动速率要高于L/γ界面,也就是说,包晶转变过程δ→γ转变占比较大。相关文献[10~15]表明,亚包晶钢在连铸时出现的一系列问题主要与凝固过程中发生包晶转变δ→γ引起的体积收缩大有关。工业上,经常采用高碱度保护渣、热顶结晶器以及降低结晶器冷却强度等措施来控制亚包晶钢连铸问题,但是对于这些调控措施一直缺乏一个合理的机理解释。
本工作采用超高温激光扫描共聚焦显微镜,对不同冷却速率下15CrMoG亚包晶钢包晶凝固过程进行原位动态观察,并结合包晶相变热力学和动力学研究,分析15CrMoG钢包晶凝固特征和机制。
1 实验方法
实验用钢取自于15CrMoG热轧棒材,化学成分(质量分数,%)为:C 0.14,Si 0.21,Mn 0.54,Cr 0.99,Mo 0.43,P 0.008,S 0.005,Fe余量。将15CrMoG钢机械加工成直径为7.6 mm、高为3 mm的圆柱体。然后用SiC砂纸和金刚石抛光膏对圆柱试样进行打磨处理。为了防止污染熔化钢液,试样在放入超高温激光共聚焦显微镜加热炉之前,用超声波清洗仪清洗5 min,烘干后放入金相加热炉,炉内通入高纯Ar气体加热。
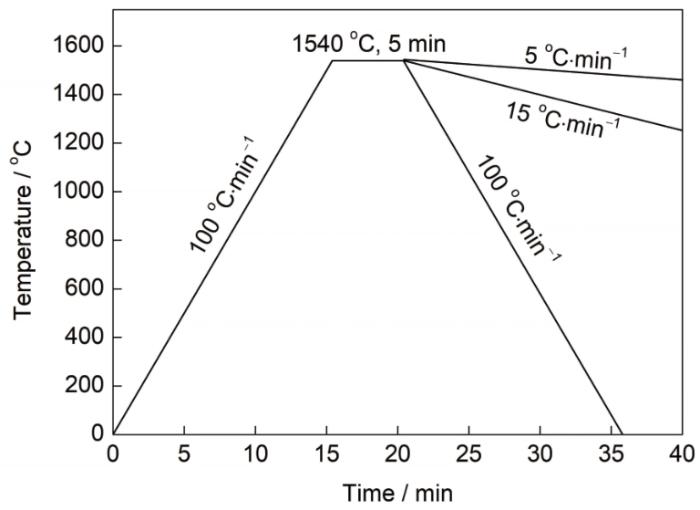
实验中使用的温控制度如图1所示。试样以100 ℃/min升温速率由室温升至1540 ℃,保温5 min,待观察试样完全熔化后以不同的冷却速率(5、15和100 ℃/min)冷却至室温,利用VL2000DX激光共聚焦扫描显微镜的成像系统,原位动态地观察钢液的整个包晶凝固过程,并存储为视频文件。
图1
2 实验结果
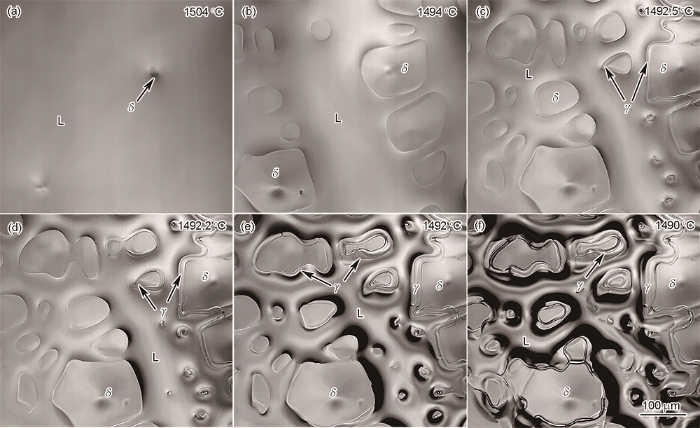
图2
图2
冷却速率为5 ℃/min 时δ相析出和包晶相变原位观察
Fig.2
In situ observations of δ precipitation and peritectic phase transformation at a cooling rate of 5 ℃/min (L—liquid phase)
(a, b) precipitation and growth of δ-ferrite (c, d) peritectic reaction (e, f) peritectic transformation
图3
图3
冷却速率为15 ℃/min时δ相析出和包晶相变原位观察
Fig.3
In situ observations of δ precipitation and peritectic phase transformation at the cooling rate of 15 ℃/min (a, b) precipitation and growth of δ-ferrite (c, d) peritectic reaction (e, f) peritectic transformation
图4
图4
冷却速率为100 ℃/min时δ相析出和包晶相变原位观察
Fig.4
In situ observations of δ precipitation and peritectic phase transformation at the cooling rate of 100 ℃/min
(a, b) precipitation and growth of δ-ferrite (c, d) peritectic reaction (e, f) peritectic transformation
图5和6分别为不同冷却速率凝固条件下的包晶反应和包晶转变。冷却速率为5和15 ℃/min时均可以观察到包晶相γ沿着L/δ界面生长(图5a和b);而冷却速率为100 ℃/min时并未观察到此现象,并且γ相在δ相界面形成的包晶层比较薄。另外,冷却速率为5 ℃/min时,在L/δ/γ三相交界点出现了明显的凹陷,如图5a所示,这是δ相局部重熔所致[16]。Griesser等[7]认为包晶反应过程δ相重熔是δ/γ/L三相交界处L/γ和L/δ界面存在温度差异所致。但是,冷却速率为15 ℃/min时,包晶相γ沿着L/δ界面生长并未观察到δ相重熔现象。这是因为冷却速率增加加快了包晶反应速率,δ相重熔量少,难以观察到。
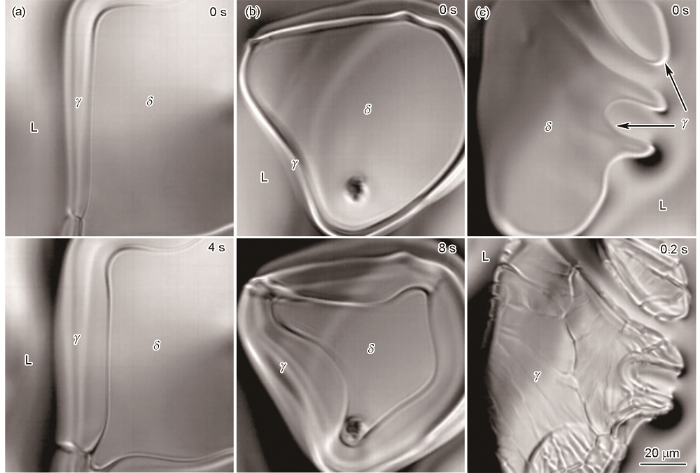
图5
图5
不同冷却速率条件下的包晶反应
Fig.5
Peritectic reactions at cooling rates of 5 ℃/min (a), 15 ℃/min (b) and 100 ℃/min (c)
图6
图6
不同冷却速率条件下的包晶转变
Fig.6
Peritectic transformations at cooling rates of 5 ℃/min (a), 15 ℃/min (b) and 100 ℃/min (c)
3 分析讨论
3.1 包晶反应
图7
图7
L/δ界面浓度分布示意图
Fig.7
Schematic of the concentration distribution at the
式中,内部能量的总变化(dU)与强度变量(温度T、压力P、收缩力F、电势Ψ以及化学式μi)和广度变量(熵S、体积V、收缩长度l,电量e以及粒子数量Ni)变化的乘积有关[20],其中,i为变量,n为变量上界。根据所考虑的特定系统,可以添加一些附加项。当系统与环境的能量交换过程中,由引力、电场以及磁场引起的系统的外部势能保持恒定时,该系统剩余能量的变化量为其内能U的变化量dU:
由于S、V以及Ni是广义变量,Euler Homogeneous函数定理使dU可以简单地积分为:
在一定的温度和压力条件下,从热力学系统中获得有用或者可用的能量称为Gibbs自由能(G),定义为:
将
随着冷却速率增加包晶相γ形核过冷度增大,形核热力学驱动力增加,导致包晶反应动力学更高,包晶反应速率更快,因此,冷却速率为5和15 ℃/min时包晶反应速率慢,可以观察到包晶相γ沿着L/δ界面生长;而100 ℃/min时,包晶反应速率非常快,观察不到包晶相γ沿着L/δ界面生长现象。
3.2 包晶转变
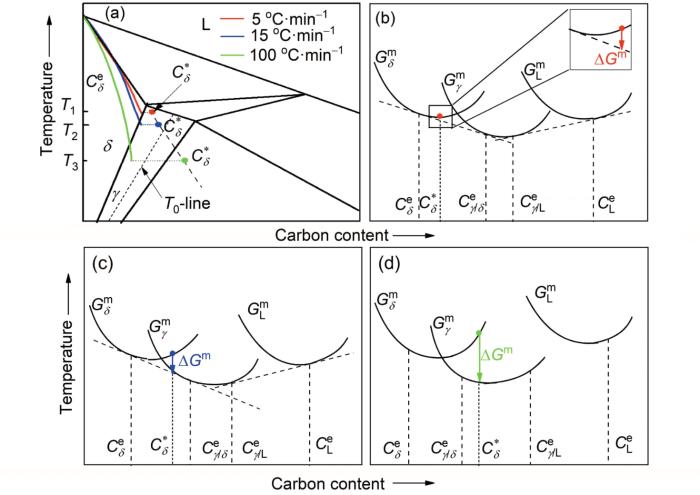
图8
图8
不同冷却速率条件下C浓度分布与Gibbs自由能
Fig.8
Carbon concentration distribution (a) and Gibbs free energies at cooling rates of 5 ℃/min (b), 15 ℃/min (c) and 100 ℃/min (d), respectively (T1, T2 and T3 are peritectic phase transformation temperatures at cooling rates of 5, 15 and 100 ℃/min, respectively. T0-line is the thermodynamic equivalence of δ and γ. G
Color online
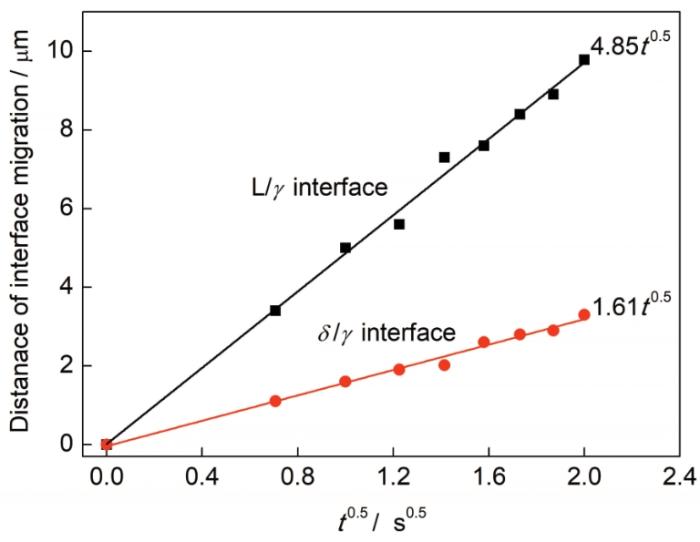
图9
图9
冷却速率为5 ℃/min条件下L/δ和δ/γ界面迁移距离与时间平方根的关系
Fig.9
Migration distances of the L/δ and δ/γ interfaces as a function of the square root of time at the cooling rate of 5 ℃/min (t—time)
冷却速率为100 ℃/min时,溶质原子在L/δ界面上形成的浓度梯度非常陡峭,包晶相变温度T3要远小于平衡包晶温度,包晶转变δ→γ热力学驱动力变得更大(图8d),导致块状转变发生。根据热力学原理,如果把一个相直接转化为另一个相,从而降低系统自由能,就会发生块状转变。对于相同的组分,发生块状转变的热力学条件是产物相的Gibbs自由能小于母相的Gibbs自由能。这种转变的临界极限是同素异形相边界,如图8a中T0-line所示。在同素异形相边界上,δ相和γ相具有相同的组分,同时具有相同的Gibbs自由能[23,24]。如图8a所示,冷却速率为5和15 ℃/min时发生包晶相变温度高于T0-line,所以没有发生大规模δ→γ转变。而冷却速率为100 ℃/min时包晶反应温度低于C
块状转变速率由转变界面过程控制,是一个晶格到另一个晶格的原子顺序的重构,与扩散控制转变相比,这种重构速度要快很多[25]。包晶转变过程中发生块状转变,体积收缩速率较大;而发生扩散控制转变,体积收缩速率较小。在连铸时,包晶转变模式的局部差异和体积收缩速率的不同导致初生坯壳不均匀生长。降低冷却速率可以避免块状转变的发生,降低体积收缩差异,减小初生坯壳不均匀性,因此,在连铸亚包晶钢时,采用高碱度保护渣、热顶结晶器以及降低结晶器冷却强度等措施来降低钢液初始冷却速率。
4 结论
(1) 冷却速率为5和15 ℃/min时,δ相以胞状方式析出;当冷却速率增加至100 ℃/min时,δ相以枝晶方式析出。
(2) L/δ界面处浓度梯度的存在增加了包晶γ相Gibbs自由能成核势垒;随着冷却速率增加,穿过L/δ界面浓度梯度增加,包晶γ相形核所需过冷度增大,包晶反应温度降低。
(3) 冷却速率增加导致包晶反应动力学增加,包晶反应速率更快。冷却速率为5和15 ℃/min时,包晶反应速率慢,可以观察到包晶相γ沿着L/δ界面生长;而冷却速率为100 ℃/min时,包晶反应速率非常快,观察不到包晶相γ沿着L/δ界面生长的过程。
(4) 随着冷却速率增加,δ→γ转变界面呈现3种不同的模式。冷却速率为5 ℃/min时,δ→γ转化界面呈现溶质扩散控制的平面形态;冷却速率为15 ℃/min时,δ→γ转化界面呈现溶质扩散控制的胞状形态;冷却速率为100 ℃/min时,出现界面过程控制的δ→γ块状转变。
(5) 包晶转变(δ→γ)中发生块状转变,体积收缩速率较大;而发生扩散控制转变,体积收缩速率较小。在连铸时,降低冷却速率可以避免块状转变的发生,降低体积收缩差异,减小初生坯壳不均匀性。
参考文献
Origin of heat transfer anomaly and solidifying shell deformation of peritectic steels in continuous casting
[J].
Abnormal mold level fluctuation during slab casting of peritectic steels
[J].
The effect of mould flux properties on thermo-mechanical behaviour during billet continuous casting
[J].
A study about the influence of carbon content in the steel on the casting behavior
[J].
Solidification microstructures: Recent developments, future directions
[J].
Kinetics of peritectic reaction and transformation in Fe-C alloys
[J].
Mechanism of the peritectic phase transition in Fe-C and Fe-Ni alloys under conditions close to chemical and thermal equilibrium
[J].
Kinetics of the diffusion controlled peritectic reaction during solidification of iron-carbon-alloys
[J].
Solidification of iron-base alloys
[J].
Mechanism of crack formation during continuous casting of peritectic steel slabs
[J].The solidification of Ag-Zn alloy, which has similar shrinking properties to Fe-C alloy during peritectic phase transformation, was studied in this paper. The unidirectional solidification experiment was performed to investigate the solidification microstructure, the relationship between the shrinkage during peritectic phase transformation and the generation of crack on the slab was analyzed. The results indicate that the crack is mainly cause by shrinkage during perirtectic phase transformation(δ→γ) ,and for this reason, the subperitectic steel bears more crack than other kinds of steel.
包晶钢铸坯裂纹形成机理的实验研究
[J].包晶相变是包晶钢较其它碳含量钢种特有的相变,该相变由体心立方结构的δ相转变为面心立方结构的γ相,由于晶格致密度的增加,伴随该相变产生较大的体积收缩。据此选用具有相似收缩特征的Ag-Zn合金作为研究对象,进行定向凝固实验。对其凝固组织进行研究,分析了包晶相变收缩与裂纹产生之间关系,认为包晶相变过程中包晶转变(δ→γ)阶段引起的收缩是导致铸坯裂纹的主要原因,据此解释了为什么连铸生产中亚包晶钢较其它钢种裂纹严重。
Application of dilatometric analysis to the study of solid-solid phase transformations in steels
[J].
A novel approach for evaluating the contraction of hypo-peritectic steels during initial solidification by surface roughness
[J].
Influence of cooling rate on the contraction of peritectic transformation during solidification of peritectic steels
[J].Driven by the demand for the improving mechanical properties of steel products and the cost reduction in alloys, steels falling within the peritectic composition range are designed recently. However, notoriously cast surface defects such as cracks, deep oscillation mark formation and breakouts are found to occur frequently during continuous casting of steels, particularly at high casting speeds. This phenomenon is closely related to the shrinkage of phase transformation caused by the peritectic transformation. In order to understand the effects of cooling rate on the contraction of the peritectic transformation, the initial solidification processes of a peritectic steel (Fe-0.1C-0.21Si-1.2Mn, mass fraction, %) were observed using high-temperature confocal laser scanning microscopy under different cooling rates, and then variations in surface roughness were measured to reflect the degree of peritectic transformation contraction. The results show that the peritectic transformation occurs a massive transformation when the cooling rate exceeds the critical value. The massive transformation results in a sudden peritectic transformation contraction and surface roughness variations, which directly cause the occurrence of surface longitudinal cracks of slabs at high casting speeds. The contraction increases first and then decreases with the cooling rate increasing and the maximum surface roughness at the middle cooling rate (20 ℃/s) is about 2.8 times more extensive than that which occurs at the low cooling rate of 2.5 ℃/s. The phenomenon that the peritectic transformation contraction decreases under the high cooling rate may provide a new strategy to reduce cracks occurring in high speed casting.
冷却速率对包晶钢凝固过程中包晶转变收缩的影响
[J].通过高温激光共聚焦显微镜模拟观察了Fe-0.1C-0.21Si-1.2Mn (质量分数,%)包晶钢在不同冷却速率下的包晶相变过程,然后利用试样表面粗糙度变化反映了包晶转变收缩程度的不同。结果显示,冷却速率超过临界值后包晶转变能够发生快速相变,快速相变引起突然的包晶转变收缩和表面粗糙度变化。随冷却速率的增加包晶钢的包晶转变收缩呈先增加后减小的趋势,在冷却速率为20 ℃/s时表面粗糙度达到最大值,此时的表面粗糙度约是低冷却速率(2.5 ℃/s)时表面粗糙度的2.8倍。当冷却速率足够大后包晶转变收缩又开始减小,这一变化为高拉速下减少包晶钢连铸坯表面纵裂纹的发生提供了新策略。
Study of the effect of carbon on the contraction of hypo-peritectic steels during initial solidification by surface roughness
[J].
The influence of peritectic reaction/transformation on crack susceptibility in the continuous casting of steels
[J].
Kinetics of the peritectic phase transformation: In-situ measurements and phase field modeling
[J].
A solid-liquid diffusion couple study of a peritectic reaction in iron-carbon system
[J].
Diffusional constrained crystal nucleation during peritectic phase transitions
[J].The casting behaviour, microstructure formation and resulting mechanical properties of materials that undergo a peritectic phase transition during solidification are largely determined by the kinetics of this high-temperature phase transformation during the production process. However, current nucleation models do not accurately predict the nucleation behaviour and phase transition kinetics in many polycrystalline materials. Utilizing a newly developed experimental technique, we have performed in situ observations to study the nucleation behaviour of a newly forming intermediate phase by using the peritectic phase transition in Fe-C and Fe-Ni alloys as examples. In our experiments as well as by using thermodynamic and kinetic arguments we demonstrate that nucleation of a new intermediate (peritectic) phase can be constrained in the presence of solute diffusion fields that form during primary solidification due to an increase in the Gibbs free energy barrier for nucleation. We found a strong correlation between the magnitude of these diffusion fields and and the resulting nucleation undercooling required for the formation of a new phase. Our study casts new light on, and clarifies for the first time, the much-debated underpinning reason for the occurrence of massive phase transformations occurring during solidification processing at large nucleation undercoolings. These new insights contribute to the improvement of nucleation theory and allow more accurate predictions on nucleation events and, in turn, physical properties of materials that undergo phase transitions in the course of materials production processes. (c) 2014 Acta Materialia Inc. Published by Elsevier Ltd.
Effect of nucleation undercooling on the kinetics and mechanism of the peritectic phase transition in steel
[J].The mechanism and kinetics of the peritectic phase transition in steel were studied by using a concentric solidification technique in a high-temperature laser-scanning confocal microscope. Strong solute diffusion fields are established during solidification of the primary delta phase, which leads to suppression of nucleation of the austenite phase, and hence the extent to which a steel can be cooled to a temperature below the equilibrium peritectic temperature before the peritectic reaction will occur. The magnitude of these diffusion fields was also determined as a function of carbon content, alloying additions, cooling rate and the fraction of primary delta phase that solidified before the peritectic reaction occurred. Depending on the extent of undercooling required to nucleate the first austenite platelet on the liquid/delta-ferrite interface, three different modes of the solid-state peritectic transformation of delta to austenite have been observed: a diffusion-controlled phase transformation that occurred by the progression of a planar interface; a diffusion-controlled transformation with cellular/dendritic morphology; and a massive-type of transformation. Under certain circumstances, a changeover between these modes has also been observed. The underpinning thermodynamic considerations that lead to these different modes of transformation are discussed and a new explanation is provided for the occurrence of a massive transformation of delta-ferrite to austenite. The relevance to and implications of these new findings to the continuous casting of steel of near-peritectic steel composition are also discussed. (C) 2014 Acta Materialia Inc. Published by Elsevier Ltd.
Nonequilibrium Thermodynamics: Transport and Rate Processes in Physical, Chemical and Biological Systems
[M]. 2nd Ed.,
Analysis of solute distribution in dendrites of carbon steel with δ/γ transformation during solidification
[J].
Solute trapping-free massive transformation at absolute stability
[J].Directional transformation of a hypo-peritectic Fe-17.5 at.% Co alloy was studied. Two consecutive phase transformations solidification (liquid to delta ferrite) and solid-state transformation (delta ferrite to gamma austenite)-were observed and compared with theory. In all experiments, the solidification front was planar and in the steady-state, and therefore produced a homogeneous parent phase for the following delta-gamma transformation. Depending on the growth conditions, gamma transformed from delta as cells or as a plane front. The cell tip radius decreased with growth rate from V = 1-5 mu m s(-1). At higher velocities, between 7 and 10 mu m s(-1) the delta/gamma interface morphology became planar. In order to explain this morphological transition, volume diffusion-controlled plane front growth and dendrite growth theory was applied. Good agreement was obtained between theory and experiments. It is concluded that plane front stabilization with increasing velocity is due to absolute stability, with a concentration spike at the transformation front. In the steady-state, this leads to composition invariance, typical for massive transformation. Computed interface velocities for quenching in heat treatment, which can be as high as several centimeters per second, show that, in certain cases, the controlling mechanism of massive transformation is steady-state plane front growth with a narrow concentration spike and not complete solute trapping. (C) 2010 Acta Materialia Inc. Published by Elsevier Ltd.