钢铁工业是国民经济的主要基础产业,在世界各国的经济发展中发挥着巨大的作用。我国钢铁工业为我国国民经济的快速发展作出了重大贡献,也为世界钢铁工业的发展起到积极的促进作用[1]。2022年中国粗钢产量约为10.1 × 108 t,约占世界粗钢产量的55.2%,持续保持世界首位。从20世纪50年代以来,现代钢铁生产流程已发展成为炼钢→精炼→连铸→成型工艺的优化组合,其中连铸作为承上启下的重要环节,对高品质钢的生产效率、质量和经济性至关重要[2]。2022年,中国连铸钢产量占粗钢产量的98.4%。在连铸过程中,中间包是衔接钢包和结晶器的一个过渡容器,它的主要功能有:分流作用;连浇作用;降低中间包内钢液的静压力,减少对结晶器中初生坯壳的冲刷;夹杂物上浮和去除[3]。随着交通运输、能源化工和机械制造等重点行业对钢铁材料需求的不断增加,对钢铁材料纯净度的要求也越来越高[4]。中间包作为钢液进入结晶器前所经过的最后一个具有耐火材料的反应器,末端冶炼对钢铁产品的质量起到至关重要的作用。钢液流经中间包发生的二次氧化现象会对钢中非金属夹杂物的成分、尺寸和数量密度产生影响,并且可能堵塞水口,最终对钢铁产品的性能产生不利的影响[5~7]。造成钢液二次氧化的主要原因来自大气、中间包覆盖剂和耐火材料(图1[8]),为了更加明确钢液流经中间包过程中发生的二次氧化对钢液的影响机理,本文系统地总结了不同钢种中间包内钢液二次氧化的影响因素及二次氧化对钢液成分和非金属夹杂物特征的影响规律,分析了控制中间包中钢液二次氧化的措施,同时对建立数学模型研究中间包内钢液二次氧化行为对非金属夹杂物的影响进行了展望。
图1
1 中间包内钢液二次氧化的来源
1.1 大气对中间包中钢液二次氧化的影响
在钢液初始浇注和稳定浇注阶段,钢液在中间包中的二次氧化行为并不一致[9]。在初始浇注阶段,中间包入口处出现液面裸露,也称“红眼”(open eye)[10],导致钢液严重吸气,进而发生二次氧化,造成钢液的纯净度变差;在稳定浇注阶段,钢液表层的空气和中间包覆盖剂是造成钢液二次氧化的主要因素。Tanak等[11,12]分析了大气环境下中间包中钢液的二次氧化行为,指出中间包中钢液二次氧化的最主要原因来自大气,而随着浇注过程的进行,中间包覆盖剂对钢液造成的二次氧化则成为主要原因。Sasai和Mizukami[9]得出了类似的结论,指出钢液开始浇注的过程中,钢液表面大气、钢包内衬填料、中间包入口处和中间包覆盖剂对钢液造成的二次氧化反应都是同时进行的,如图2[9]所示,其中在中间包入口处造成的二次氧化占66%。在稳定浇注过程中,中间包中钢液的二次氧化主要受到钢液表面大气和中间包覆盖剂的影响。Sasai和Mizukami[13]还研究了中间包内钢液的搅动对二次氧化的影响,发现静态条件下铝镇静钢的二次氧化反应的限制性环节为O2在氧化膜中的扩散,而在搅动状态下则转变为O2在气相中的传质。钢液流经中间包内时,为了抑制由于吸气造成的钢液二次氧化现象,特别是钢液从钢包经过长水口进入中间包的过程,通常向中间包内吹入惰性气体,如Ar气,以隔绝大气中的O2与钢液的反应。但是一般情况下,受到中间包容量和实际操作的影响,使用Ar气难以实现对中间包的完全封闭。在这种情况下,O2也可以伴随着Ar射流进入中间包内部,进而使钢液产生二次氧化[14]。钢液成分也会影响钢液对大气中O2的吸收速率,Sasai和Matsuzawa[15]发现,在实际浇注过程中,在搅动的情况下(氧势
图2
图2
造成中间包内钢液二次氧化的原因[9]
Fig.2
Factors responsible for the reoxidation of molten steel in tundish[9] (Δ[O] represents the amount of oxidation of the molten steel by the factors in the initial teeming stage and the stable casting stage)
(a) oxidation by air at the ladle shroud
(b) oxidation by air from the surface
(c) oxidation by ladle well-packing material
(d) oxidation by tundish cover powder
1.2 中间包覆盖剂对钢液二次氧化的影响
中间包覆盖剂最初的作用只是防止钢液在浇注过程中温降过大,但随着对高品质钢的需求越来越大,对中间包覆盖剂冶金功能的要求也随之增加,如钢液保温减少热量损失、隔绝钢液与大气避免钢液二次氧化、吸附和溶解钢液中非金属夹杂物、不与钢液发生化学反应避免污染钢液以及减少对中间包耐火材料的侵蚀[18,19]。因此,在实际操作过程中,双层渣的应用较为广泛[6,20,21],即底层为熔点较低的碱性覆盖剂,顶层为碳化稻壳灰(rice husk ash,RHA)。在浇注稳定阶段,由于钢液热量的传输,根据中间包内双层渣的物理状态可以将渣层分为3个区域:靠近温度较高的钢液一侧为完全液相渣区域、顶层温度较低且含有大量高熔点碳化稻壳灰的固相渣区域、处于完全固相渣和液相渣中间区域的固-液相渣共存的区域。底层的液相渣区域主要起到吸收和溶解钢中非金属夹杂物的作用,而顶层的固相渣以及固-液相渣共存区域则起到保温和隔绝大气的作用。但是,在实际操作过程中发现,顶层固相碳化稻壳灰中的SiO2不断地向下层熔渣中溶解和扩散,致使在渣-钢界面反应区域熔渣中SiO2的含量不断升高,造成钢液的二次氧化和夹杂物的生成。底层熔渣的黏度往往是影响SiO2溶解速率和机理的主要因素[22]。本课题组[23]最近提出了渣的夹杂物容量(inclusion capacity of slag)概念,即Zh指数,用来预报非金属夹杂物在渣中的溶解速率,其表达式如
式中,Ci 和Ci, saturation分别表示渣中含有夹杂物相i的质量分数和渣中该物相的饱和质量分数(%),dp, 0为夹杂物初始直径(m),ρslag和ηslag分别代表熔渣的密度(kg/m3)和动力学黏度(kg/(m·s)),g为重力常数(m/s2)。从
为了明确中间包覆盖剂中SiO2对铝镇静钢的二次氧化反应行为的影响,Basu等[20]在浇注含Ti铝镇静超低碳钢时,在中间包使用底层碱性覆盖剂成分为(46~50)CaO-(42~45)Al2O3-(0~3)SiO2-(0~2)Fe2O3-(1.8~2.5)TiO2 (质量分数,%,下同)和顶层为碳化稻壳灰(SiO2 > 94%)的双层渣,发现在底层与钢液接触的熔渣中SiO2含量增加,并与镇静钢中Al和Ti元素发生氧化还原反应,生成Al2O3和TiOx -Al2O3夹杂物,如
Goto和Miyazawa[26]对比了从钢包至中间包连铸过程中,渣中SiO2对非脱氧钢和铝脱氧钢二次氧化行为的影响,发现非脱氧钢中的Si和溶解氧含量均增加,铝脱氧钢中只有Si含量增加而溶解氧与Al反应生成Al2O3夹杂物,夹杂物上浮去除使得总氧(T.O)含量变化不大。Bessho等[27]发现,钢液中Al还原中间包SiO2-CaO-Al2O3渣中SiO2的反应速率取决于渣中SiO2活度(
图3
表1 中间包覆盖剂造成钢液二次氧化的研究总结[8,20,21,24~29,31~34]
Table 1
| Tundish cover powder | Steel composition | Experimental method | Ref. |
|---|---|---|---|
| (39.5-62.5)CaO-(2.7-22.2)Al2O3-(2.6-47.4)SiO2 | Ultra-low carbon steel | The industrial trials were carried out at the No.5 continu-ous caster at Mizushima Works in Kawasaki Steel Corp-oration | [27] |
52CaO-35Al2O3-13SiO2 50CaO-50SiO2-100SiO2 | Al-killed steel | Reoxidation of Al-killed steel by slag and air was investigated in laboratory experiments and industrial trials in 85 t tundish at Sumitomo Metal Industries | [31] |
| * | Non-killed and Al-killed steels | The industrial trials were conducted in 60 t tundish at Nippon Steel Corporation | [26] |
RHA + (46-50)CaO-(42-45)Al2O3-3SiO2-2Fe2O3- (1.8-2.5)TiO2 | Ti-bearing Al-killed ultra-low carbon steel | The industrial trials were conducted at Tata Steel | [20] |
26.8CaO-42.3Al2O3- 27.6SiO2 | Ti-stabilized ultra-low carbon steel | 45 g steel covered with 40 g tundish slag was held in an alumina crucible at 1823 K | [24] |
RHA + 45CaO-35Al2O3- 10SiO2-5MgO-5CaF2 | Al-killed Fe-C-Si steel | 500 g of steel and 50 g of slag were placed in a MgO crucible and heated to 1823 K (R = 0.14, 0.26, 0.38, and 1.0) | [8] |
RHA + 55CaO-35Al2O3- 4SiO2-4MgO | Al-killed Fe-C-Si steel | 500 g of steel and 50 g of slag were placed in a MgO crucible and heated to 1823 K (RCA = 0.5, 0.75, 0.83, 0.87, and 0.90) | [28] |
50CaO-30Al2O3-10SiO2- 10MgO-(5, 10)Cr2O3 | Al-killed YT01 steel | The Al-killed steel was held in a MgO crucible at 1923 K for 30 min and then 20 g of slag with various Cr2O3 contents was added | [29] |
| 73CaO-25SiO2-(1-15)FeO-1MnO | Ultra-low carbon steel | The slag-metal reactions between 30 g pre-melted slag and 100 g of sample were conducted in a zirconia crucible at 1853 K | [25] |
44.2CaO-44.1Al2O3- 6.0SiO2-2.68MgO-1.17TiO2 | Ti-bearing Al-killed ultra-low carbon steel | 400 kg of the basic tundish flux was added to the surface of the molten steel in the 70 t tundish, and then 60-80 kg of rice hull ball was added to the tundish flux | [21] |
| RHA + 45.65CaO-22.88Al2O3-20.75MgO-2.84SiO2 | Si-Mn killed SAE 1055 steel | 150 g steel and 20 g tundish slag (5 g RHA) were acco-mmodated in an Al2O3 crucible at 1853 K | [32] |
| 53.5CaO-41.5Al2O3-5MgO and 47.5CaO-47.5SiO2-5MgO | Si-killed 304 stainless steel | 600 g of 304 stainless steel and 50 g of slag were cont-ained in a MgO crucible at 1773 K | [33] |
RHA, RHA + 51.1CaO- 43.3SiO2, and RHA + 52.6CaO-40.7Al2O3 | Si-killed 316L stainless steel | 600 g of 316L stainless steel was placed in a MgO crucible at 1773 K and then 45 g of tundish flux was quickly added to the surface of the molten steel | [34] |
Duan等[34]报道了不同种类的中间包覆盖剂,如RHA、RHA + CaO-SiO2 (RHA + Flux A)和RHA + CaO-Al2O3 (RHA + Flux B),对硅脱氧316L不锈钢二次氧化行为的影响。结果表明,当使用RHA和RHA + Flux A覆盖剂时,钢液发生严重的二次氧化,且随着反应时间延长,钢中Si和Mn含量分别增加和降低,反应如
式中,[%Mn]0和[%Mn]t 分别表示钢液中初始Mn含量和任意反应时间t时钢液中的Mn含量(质量分数,%),[%Mn]e为反应(5)达到平衡时钢液中的Mn含量(质量分数,%),A和Vm分别代表渣/钢反应界面的面积(m2)和钢液体积(m3)。根据Duan等[34]研究可以得到km,Mn = 6.4 × 10-6 m/s (1773 K)。
Alves等[32]比较了CaO-Al2O3基覆盖剂和RHA + CaO-Al2O3双层覆盖剂对Si-Mn镇静钢二次氧化行为的影响,指出仅使用CaO-Al2O3基覆盖剂就可以提高钢液的纯净度。Kim等[33]分析了53.5CaO-41.5Al2O3-5MgO和47.5CaO-47.5SiO2-5MgO覆盖剂对硅脱氧304不锈钢纯净度的影响,发现CaO-Al2O3-MgO能够增加304不锈钢的纯净度,CaO-SiO2-MgO则对不锈钢的纯净度基本没有影响。可以发现,虽然顶层为RHA和底层为碱性覆盖剂的双层渣可以在一定程度上或短时间内防止钢液在中间包中热量的耗散并起到吸收夹杂物的作用,但是顶层的RHA中的SiO2会向底层渣中扩散,造成底层渣中SiO2含量增高,导致钢液中的易氧化元素(如Al、Ti和Mn)与渣中SiO2发生二次反应(如式(
1.3 中间包耐火材料对钢液二次氧化的影响
水口包括长水口(ladle shroud)和浸入式水口(submerged entry nozzle,SEN),是分别连接钢包和中间包以及中间包和结晶器并起到保护性浇注作用的重要耐火材料,可以起到防止钢液飞溅和卷渣等多重作用[35]。超低碳铝镇静钢在浇注时会产生严重的水口结瘤和堵塞[5],其中一个原因就是,在浇注温度下(1803~1823 K),水口材料中的氧化物会发生碳热还原反应生成CO和SiO气体[36~38],并与钢液中的Al和Ti反应生成二次氧化产物,进而恶化钢液的可浇性[39]。Fukuda等[37]在1873 K下使用旋转柱侵蚀法将直径为30 mm的Al2O3-C耐火材料浸入钢液中4 h,发现在耐火材料表面有网状的Al2O3夹杂物生成,这种网状夹杂物的生成是由SiO与Al2O气体和钢中Al反应造成的。Sasai和Mizukami[40]计算了浸入式水口中SiO2与Al2O3发生碳热反应生成气体SiO、Al2O和AlO的平衡氧分压,发现不同氧化性气体的平衡氧分压的大小排序为
图4
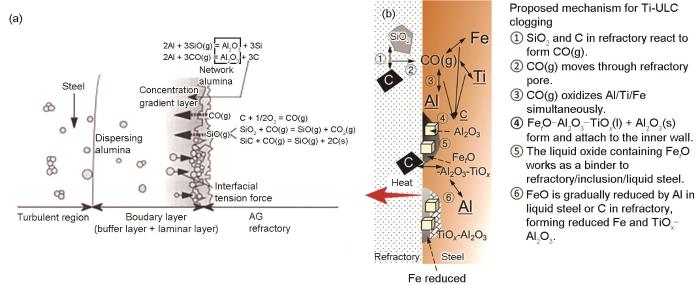
Lee等[44]在1833 K下使用CO气体(0.5 L/min)喷吹钢液表面30 min,用来模拟含Ti铝镇静钢在浇注过程中水口材料发生碳热反应产生的CO对钢液二次氧化行为的影响,反应机理如图4b[44]所示。在浇注含Ti铝脱氧超低碳钢时,水口耐火材料发生碳热反应生成的CO (反应(7))扩散至钢液与耐火材料界面,CO与钢液中Al、Ti和Fe同时反应,生成液态Fet O-Al2O3-TiOx (反应(9))和固态Al2O3夹杂物(反应(8))并附着在水口耐火材料内壁,液态Fet O-Al2O3-TiOx 中的Fet O被钢液中的Al或者水口耐火材料中的C还原,最终水口耐火材料的沉积物转变为还原的Fe液滴和Al2O3-TiOx 复合氧化物[44]。Lee和Kang[45]还报道了当水口耐火材料中含有CaO、ZrO2和SiO2时,它们会较快地溶解进生成的Fet O-Al2O3-TiOx 液态沉积物中,最终转变为Fe液滴和CaO-Al2O3-TiOx -ZrO2-SiO2水口沉积物。
在连铸过程中,中间包工作面与熔渣和钢液接触并承受着熔渣的化学侵蚀和钢液的物理冲刷等作用,会导致中间包耐火材料(主要成分为MgO-SiO2)出现局部损毁,进而影响钢液的纯净度和中间包的正常使用[46,47]。采用喷补技术对中间包耐火材料损毁处进行维护,可以延长中间包使用寿命[32,48]。中间包喷补料(gunning material,GM)主要分为MgO和Al2O3基耐火材料,杂质成分主要含有SiO2和FeO。Yan等[49]比较了3种喷补料MgO、Al2O3以及MgO + MgO·SiO2对钢液二次氧化行为的影响。通过电子探针(EPMA)对耐火材料与钢液界面的观察发现:(1) 钢液会渗入进喷补料中;(2) 耐火材料与钢液界面处发现喷补料剥落形成的大尺寸颗粒状外来夹杂物;(3) 钢液发生二次氧化现象(
图5
图5
钛稳定超低碳钢和耐火材料界面显微组织[49]
Fig.5
Microstructure of the interface between Ti-stabilized ultra-low carbon steel and MgO-based gunning material[49] (P
(a) oxygen from refractory (trapped air in pores, reducible FeOx and SiO2 oxides)
(b) molten steel with a low oxygen content (around 10-11 Pa)
(c) FeOx at the refractory/steel interface
表2 中间包耐火材料导致钢液二次氧化的研究总结[24,32,49,51~55]
Table 2
| Tundish refractory | Steel composition (mass fraction / %) | Main conclusion | Ref. |
|---|---|---|---|
Al2O3-SiO2 | Ni-Fe alloy | The substitute of mullite to SiO2 mullite of the refractory bonding matrix or the use of alumina bricks can avoid the reoxidation of the melt and intense inclusion formation | [52] |
| GM, MgO boards, and dry powder | 0.45C-3.1Si-0.4Mn-8.5Cr-0.25Ni | An oxidized steel layer can be formed at the steel/refractory lining interface | [53] |
MgO, Al2O3, MgO + 2MgO·SiO2 GMs | Ti-stabilized ultra-low carbon steel | The large difference in oxygen potential between refractory and steel phase leads to the formation of (Mg, Fe)O layer ( | [49] |
| MgO- and Al2O3-based GMs | Ti-stabilized ultra-low carbon steel | The oxidation capacity of MgO GM with 10SiO2-6FeO was higher than that of Al2O3 GM with 3.3SiO2 + 2FeO | [24] |
| MgO-CaO and MgO-based GMs | Si-Mn-killed SAE 1055 steel | The GM with the reducible oxides SiO2 and FeO was responsible for providing oxygen and causing reoxidation of the molten steel | [32] |
| MgO- and Al2O3-based GMs | Ti-stabilized ultra-low carbon steel | The MgO GM represented a stronger oxidizing capacity, while Al2O3 can improve the cleanliness of the molten steel | [51] |
| High-silica tundish refractory (66MgO-27SiO2-4FeO-3CaO) | Fe-2Al alloy | The content of the oxidizing oxides in the refractory should be reduced to avoid the loss of Al in the alloy |
钢包引流砂(ladle filler sand,LFS)作为滑动水口的添加剂起到钢包自动开浇的重要作用,常用的引流砂成分为硅质、铬质、锆质和镁质。高锰高铝钢中的Mn和Al会还原引流砂(29.4Cr2O3-4.93MgO-10.7Al2O3-16.96Fe2O3-34.29SiO2)中的不稳定氧化物Cr2O3、MnO和FeO,造成钢液二次氧化,并且氧化产物使引流砂产生更多以MnO-Al2O3-FeO-SiO2为主的液相,恶化钢包引流砂的烧结性能,进而导致钢包无法自开[56]。引流砂中的Cr2O3、MnO和Fe2O3会造成轴承钢中Al元素的烧损并生成大量尺寸大于5 μm的Al2O3夹杂物,使得轴承钢中初始夹杂物类型从MgAl2O4尖晶石转变为富Al2O3尖晶石[57]。同时,某些MnO/FeO大型夹杂物也被证明来自铬质引流砂(铬铁矿相和石英相)及其烧结产物,一旦引流砂进入钢液中,想在中间包内完全去除是十分困难的[58]。钢包下渣也会造成中间包内钢液的二次氧化,应当采取相应的监控措施或优化钢包底部结构,进而减少氧化性精炼渣对钢液的二次氧化[7,59]。
2 钢液二次氧化对钢中非金属夹杂物的影响
钢液的二次氧化不仅会造成钢液成分的波动,还会造成钢中非金属夹杂物形貌、成分和数量密度的变化。Yang等[60]在调研铝脱氧钙处理API-X70管线钢的连铸过程时发现,当连铸至70 t时,钢液吸气造成钢液的二次氧化,钢液T.O含量和N含量分别从RH炉(Ruhrstahl-Heraeus degasser)出站后的14 × 10-6和31 × 10-6增加至21 × 10-6和35 × 10-6。钢液二次氧化的夹杂物成分的变化如图6[60]所示。可以看出,经过钙处理后钢中夹杂物主要成分为液态钙铝酸盐(CaO / Al2O3含量比值 = 0.86),但是钢液发生二次氧化后中间包钢液夹杂物则转变为固态钙铝酸盐CaO·2Al2O3和CaO·6Al2O3 (CaO / Al2O3含量比值 = 0.16)。由于富Al2O3的钙铝酸盐夹杂物与钢液有较强的界面能[61],因此与纯Al2O3夹杂物相比,CaO·2Al2O3和CaO·6Al2O3夹杂物更容易碰撞聚集形成较大颗粒的夹杂物,将会造成严重的水口结瘤[62,63]。诸多学者[64~67]也都报道了类似的结果,并发现铝脱氧钙处理钢发生二次氧化后,钢中夹杂物类型的转变同时受钢中S和Ca含量的影响。当钢中总钙(T.Ca)含量为10 × 10-6时,夹杂物的转变行为为CaS→液态夹杂物→CaO·2Al2O3→CaO·6Al2O3→Al2O3;而当钢中T.Ca = 30 × 10-6时,钢中CaS夹杂物全部通过反应(12)和(13)[68]分解并转变为固态钙铝酸盐夹杂物(CaO·2Al2O3 + CaO·6Al2O3)。
图6
Wang和Liu[69]发现,在含稀土Ce的铝镇静低合金钢的浇注过程中,钢液在浸入式水口位置吸气导致钢液发生二次氧化,导致钢液中的T.O含量和总氮(T.N)含量分别从11 × 10-6和41 × 10-6增加至72 × 10-6和169 × 10-6,而钢液中Ce含量从0.196%降低至0.190%。钢液二次氧化致使钢中细小的球形Ce2O2S夹杂物通过
图7
表3 钢液二次氧化导致的不同钢种中夹杂物成分转变的研究总结[8,28,34,44,60,64,69~81]
Table 3
| Steel composition | Initial inclusion | Inclusion composition after reoxidation of molten steel | Ref. |
|---|---|---|---|
Al-killed and Ca-treated API-X70 pipeline steel | Liquid calcium-aluminate inclusion | Al2O3-rich inclusion | [60] |
| Al-killed and Ca-treated 42CrMo steel | Liquid CaO-MgO-Al2O3 | Solid CaO-MgO-Al2O3 and MgO-Al2O3 | [70] |
| Al-killed Fe-0.2C-0.1Si steel | Liquid CaO-MgO-Al2O3 | Al2O3-rich inclusion | [8,28] |
| Al-killed molten steel | CaO-CaS (Al2O3) | CaO-Al2O3 (CaS) | [64] |
| Al-killed GCr15 bearing steel | CaO-Al2O3-MgO-CaS | The increase in CaO and Al2O3 contents and decrease in MgO and CaS contents of the inclusion | [71] |
| Al-killed low alloy steel containing rare earth Ce | Spherical Ce2O2S inclusion | Clustered-like CeAlO3 | [69] |
Al-killed and Ca-treated AH36 steel | Liquid CaO-Al2O3-MgO | CaO-2Al2O3 and MgO-Al2O3 | [72] |
| Ca-treated carbon-manganese steel | Liquid inclusion + CaS | Semi-solid 60.4Al2O3-25.3CaO-7.2 SiO2-7.1MgO | [73] |
| Al-killed and Ca-treated stainless steel | CaO-Al2O3-SiO2-MgO | MnO-Al2O3-SiO2-CaO | [74] |
| GCr15 bearing steel | Liquid calcium-aluminate inclusion | CaO-6Al2O3 and Al2O3 | [75] |
| Fe-Al-Ti-O melt | Al2O3 and Al2TiO5 | Ti3O5 (high oxygen potential) Al-Ti complex oxide (low oxygen potential) | [76] |
| Al-killed and Ti-alloyed IF steel | Al2O3 | Al2O3-TiOx | [77] |
| Ti-added ultra-low carbon steel | Al2O3 | Liquid Fet O-Al2O3-TiOx | [44] |
Al-killed stainless steel (20Cr-0.15Al-0.2Ti) | CaO-Al2O3-MgO | Solid Al2O3-Cr2O3-TiO2 | [78] |
Si-killed stainless steel (18Cr-8Ni-0.48Si-1.06Mn) | Al2O3-SiO2-CaO-MnO | Al2O3-SiO2-CaO-MnO with high SiO2-MnO content | [79] |
| Si-killed spring steel | CaO-SiO2 and Al2O3-SiO2-CaO | Al2O3-SiO2-MnO and SiO2-MnO | [80] |
| Si-killed stainless steel | CaO-SiO2-MgO-Al2O3 | Liquid + Mg[Al,Ti]2O4 composite inclusions | [81] |
| Si-killed 316 stainless steel | SiO2-MnO | SiO2-rich inclusion | [34] |
含Ti超低碳钢液中Al和Ti含量约在0.01%~0.1%的范围之内,如图8[82]所示,此时钢中溶解氧含量较低且可以稳定存在的夹杂物为Al2O3 (Region A)。若钢液暴露在任何可能存在的氧化性环境下,如大气或者水口耐火材料释放的CO (CO(g) = [C] + [O]),造成钢液中溶液氧含量增加,夹杂物成分则由固态Al2O3 (Region A)转变为液态Fet O-Al2O3-TiOx 夹杂物(Region B)。Lee等[44]在感应炉中使用CO气体与Fe-(0.0125, 0.025, 0.05)Al-0.05Ti合金的反应(1560 ℃)来模拟含Ti超低碳不锈钢在浇注过程中由于水口材料释放CO造成钢液二次氧化从而形成水口结瘤的现象,通过理论模拟和实验结果得出,水口耐火材料和钢液的溶解氧含量最高可以达到约500 × 10-6 (pCO = 105 Pa,1540 ℃),约是钢液内部溶解氧含量(约20 × 10-6)的20倍。从图8[82]可以看出,在此高溶解氧含量条件下,钢中稳定的夹杂物为液态的Fet O-Al2O3-TiOx,而该液态夹杂物与水口耐火材料具有良好的润湿性,因此Fet O-Al2O3-TiOx 和Al2O3的存在是含Ti铝镇静钢在连铸过程中发生水口结瘤的主要原因[45,83]。Kim等[78]指出,不锈钢(Cr含量 > 20%)中同时含有较高含量的Al (> 0.15%)和Ti (> 0.2%)时,钢中溶解氧含量会降低至约3 × 10-6。但钢液由于吸气或者钢包引流砂(Fe2O3-Cr2O3-SiO2)发生二次氧化,使得浇注过程的
图8
Li等[79]发现,在开始浇注硅脱氧不锈钢(18Cr-8Ni-0.48Si-1.06Mn)至稳定浇注前,钢液吸气造成的二次氧化使得钢液局部溶解氧含量较高而Al和Ca含量很低,钢液中夹杂物的MnO和SiO2含量增加的速率高于Al2O3和CaO含量增加的速率,由于钢中Mn含量较高,形成了直径为1~2 μm的富MnO的小尺寸颗粒状夹杂物(2[Mn] + O2 = 2(MnO))。随着钢液浇注进入稳定阶段,钢液中的小尺寸颗粒状富MnO的夹杂物作为中间产物被钢液中Ca和Al还原,伴随着夹杂物的碰撞和长大,最终钢中富MnO夹杂物消失。Lyu等[80]报道了类似的结果,发现在硅脱氧0.55C-1.32Si-0.68Mn-0.61Cr弹簧钢的浇注过程中,由于中间包内钢液的搅动吸气造成钢液二次氧化,钢液中的Si和Mn与大气中的O2反应生成MnO-SiO2夹杂物,如
在中间包覆盖剂中,RHA中过高的SiO2含量同样会造成硅镇静316L不锈钢在浇注过程中夹杂物成分发生变化。Duan等[34]使用RHA、RHA + Flux A和RHA + Flux B分别研究了中间包覆盖剂对316L不锈钢二次氧化行为的影响,实验结果如图9[34]所示。可以看出,当使用RHA和RHA + Flux A时,由于钢液发生较为严重的二次氧化(
图9
3 钢液二次氧化控制的研究进展
3.1 中间包结构优化
一个浇次非稳态浇注过程主要包括第一个钢包的开浇、换包、更换水口和本浇次最后一个钢包浇注结束。开始浇注时,钢液经过钢包长水口以较高的浇注速率首先射流到湍流抑制器底部,然后反向冲击到熔渣液面形成“渣眼”,此时钢液将会暴露在大气下造成钢液二次氧化[84,85]。考虑到冶金过程的高温特性,很难通过实验观察到钢液内部流场和温度场的分布情况[86],因此诸多学者通过数值模拟和物理模拟的方法研究利用控流装置、水口结构、水口浸入深度以及吹氩流量对由于“渣眼”形成而造成的中间包钢液二次氧化进行控制。陈宏亮[87]研究了控流装置和工艺参数对中间包内钢液多相流动的影响,指出带檐的湍流抑制器、合适的长水口浸入深度以及喇叭型长水口的结构能有效降低射流对钢液面的冲击、主流区钢液面的湍动能并预防卷渣。Wang等[85]建立了耦合钢液-渣-空气多相流动和传热的数学模型,模拟了钢液非稳定浇注阶段(钢液开浇和换包过程)钢液中溶解氧含量变化情况,结果如图10[85]所示。可以看出,初始钢液的溶解氧含量较低,但是由于非稳定浇注过程中“渣眼”打开,使钢液中溶解氧含量增加,并逐渐向中间包的两侧扩散。当钢水撞击到侧壁则向下运动,导致中间包出口处溶解氧含量较高。需要指出的是,由于建立的钢液-空气-渣-夹杂物-耐火材料和传热耦合数学模型还不完善,对整个浇注过程中钢液发生二次氧化以及对非金属夹杂物成分变化的影响还需进一步研究。
图10
3.2 覆盖剂成分优化
当浇注进入稳定状态,使用碳化稻壳灰的中间包覆盖剂对钢液的二次氧化成为主要影响因素。Xu等[21]设计了一种中间包覆盖剂(27.5 ± 7.5)CaO-(12.5 ± 5.0)SiO2-(22.5 ± 7.5)Al2O3-(12.5 ± 7.5)MgO-(7.5 ± 2.5)(Na2O-K2O)作为覆盖剂的上层渣以替换碳化稻壳灰,下层则为原先的CaO-Al2O3基覆盖剂。新设计的双层中间包覆盖剂可以抑制由碳化稻壳灰中高SiO2含量造成的钢中Al和Ti元素的损失和Si元素的增加,并降低钢液T.O含量进而提高钢液的纯净度。Kim等[33]发现,使用CaO-Al2O3基覆盖剂(53.5CaO-41.5Al2O3-5MgO)可以使硅镇静304不锈钢(18Cr-8Ni)的T.O含量比使用CaO-SiO2基覆盖剂(47.5CaO-47.5SiO2-5MgO)降得更低,但是使用CaO-Al2O3基覆盖剂可以使钢中Al含量增加,通过
Yu等[88]向CaO-Al2O3-SiO2-MgO (CaO / (Al2O3 + SiO2)含量比值 = 1.45)覆盖剂中添加碱金属氧化物(如Li2O、Na2O和K2O),研究了321不锈钢浇注过程中覆盖剂对夹杂物的吸收能力。结果表明,K2O的添加可以降低不锈钢中夹杂物的平均尺寸和数量密度,从而提高不锈钢的纯净度。Kekkonen等[89]设计了5种底层覆盖剂成分,其中上层覆盖剂为碳化稻壳灰,并在工业实验中研究底层覆盖剂成分对浇注16MnCrS5钢纯净度的影响,发现高CaO含量的70CaO-10MgO-20Al2O3和60CaO-10MgO-20Al2O3-10CaF2覆盖剂具有较强的吸收钢中Al2O3和MgO-Al2O3尖晶石类夹杂物的能力,以保证钢液浇注的连续性。以上结果通过设计中间包覆盖剂的成分提升了钢液的纯净度,如CaO-Al2O3基覆盖剂,但该覆盖剂会造成MgO基中间包耐火材料的严重侵蚀。因此,合适的覆盖剂成分和中间包耐火材料的设计是保证钢液末端冶炼纯净度应当同时考虑的。
3.3 耐火材料的选择
在浇注过程中,覆盖剂对耐火材料的侵蚀不仅是钢液中外来夹杂物的来源,而且可以改变钢中夹杂物的成分。通过设计中间包耐火材料的成分或结构可以有效地抑制钢液浇注过程中耐火材料的侵蚀,提高中间包耐火材料的使用寿命和钢液的纯净度。熔渣对耐火材料的侵蚀行为主要包括[90]:(1) 耐火材料组元直接或者间接溶解进熔渣,如形成(MgO·Al2O3)或(Mg, Fe)O/(Mg, Fe, Mn)O层[91,92];(2) 熔渣渗入耐火材料内部,造成耐火材料的抗热震性能和力学性能降低[47,93];(3) 渗入的熔渣在耐火材料内部形成新相(液相或固相),影响耐火材料的侵蚀行为[94,95]。如前文所述,高碱度中间包覆盖剂具有较高的吸收夹杂物的能力,可提高浇注过程中钢液的纯净度,此外高碱度熔渣还可以降低MgO在熔渣中的饱和溶解度,以降低覆盖剂对中间包耐火材料的化学侵蚀[96]。Zhang等[95]比较研究了CaO-MgO-SiO2渣碱度(CaO / SiO2 = 1.5~2.0)对MgO耐火材料侵蚀行为的影响,发现当熔渣碱度大于1.5时,在熔渣和耐火材料界面形成3CaO·SiO2和2CaO·SiO2高熔点固相产物层,阻碍了熔渣通过毛细作用进一步渗入到MgO耐火材料内部,同时平均晶粒尺寸为1 μm的MgO耐火材料具有更强的抗渣性。Lao等[97]指出,CaO-Al2O3-SiO2-MgO熔渣对耐火材料的侵蚀受到熔渣物理化学性质(如表面张力(γ)、黏度(η)和接触角(θ))的影响:
式中,l为熔渣浸入深度,r为耐材微孔尺寸,t为时间。Lao等[97]发现,当增加熔渣碱度和MgO含量时,微孔镁质耐火材料的抗渣性能要高于电熔镁质耐火材料,改变熔渣碱度对熔渣的γ和η的影响要大于θ,改变MgO含量则反之。Tan等[98]得到类似的结论,指出高碱度覆盖剂(CaO / SiO2含量比值 = 9.0)渗入到电熔镁质耐火材料内部并破坏耐火材料内部结构,导致MgO晶粒向熔渣中剥落和溶解。虽然微孔镁质耐火材料内部孔洞可以为熔渣提供渗入的通道,但可以使熔渣内MgO快速饱和,从而抑制微孔镁质耐火材料进一步侵蚀。他们同时向微孔镁质耐火材料内部添加ZrO2纳米颗粒,发现在MgO晶界处形成的CaZrO3-ZrO2相可以阻止熔渣向耐火材料内部渗入。Zhang等[99]研究了将微孔镁质耐火材料用作中间包过滤器对无间隙原子钢(IF钢)纯净度的影响,结果表明,耐火材料可以吸收IF钢中的Al2O3和TiO2夹杂物,并且耐火材料中的MgO与钢液中的Al和O发生反应,在界面处形成的MgO·Al2O3具有降低熔渣对耐火材料的侵蚀和降低钢液T.O含量的作用。由于微孔镁质耐火材料具有良好的隔热性能,微封闭微孔可以吸收热应力并减少耐火材料内部裂纹的萌生和扩展,因此微孔镁质耐火材料用于中间包具有良好的应用前景[47,100]。
4 结论与展望
(1) 在钢液连续浇注过程中,造成钢液二次氧化的主要原因有:钢液吸气、中间包覆盖剂和耐火材料。在非稳定浇注阶段,钢液吸气是造成钢液二次氧化的主要原因;在稳定浇注阶段,钢液表层的空气和中间包覆盖剂则成为主要原因。
(2) 目前双层中间包覆盖剂结构被广泛使用,即上层为碳化稻壳灰,下层为熔点较低的碱性覆盖剂,以使钢液保温减少热量损失、隔绝钢液与大气避免钢液二次氧化、吸附和溶解钢液中非金属夹杂物。但是碳化稻壳灰中高含量的SiO2向下层熔渣中不断地溶解和扩散,导致下层覆盖剂中SiO2活度增加并在渣/钢界面发生自溶解反应((SiO2) = [Si] + 2[O])造成钢液发生严重的二次氧化,致使钢液中的易氧化元素损失、Si元素含量增加。
(3) 中间包耐火材料和钢包引流砂中含有的不稳定氧化物FeO、SiO2、MnO和Cr2O3可以导致钢液发生二次氧化。在钢液浇注过程中,Al2O3-SiO2-C质水口材料发生碳热反应生成CO氧化性气体,并穿过内部空隙到达水口内壁与钢液接触,CO与钢液中的Al、Ti和Fe等元素同时反应生成结瘤物,这是造成水口堵塞的主要原因。
(4) 由于钢液吸气造成二次氧化,钙处理铝镇静钢的夹杂物成分由液态的钙铝酸盐夹杂物转变为固态的CaO·2Al2O3和CaO·6Al2O3或Al2O3夹杂物(取决于钢中的T.Ca含量);硅脱氧钢夹杂物中的SiO2-MnO含量增加;铈处理钢中的夹杂物由细小的球形Ce2O2S夹杂物转变为团簇状CeAlO3夹杂物。由于水口耐火材料碳热反应释放的氧化性气体CO造成耐火材料内壁和钢液界面溶解氧含量增加,超低碳含Ti铝镇静钢中夹杂物由纯Al2O3夹杂物转变为液态Fet O-Al2O3-TiOx 夹杂物;由于中间包上层覆盖剂碳化稻壳灰中的SiO2含量高,造成硅脱氧316L不锈钢中液态SiO2-MnO夹杂物转变为纯SiO2夹杂物。
通过对中间包内钢液发生二次氧化的机理分析,今后可以从以下方向对控制钢液纯净度和减少钢液二次氧化进行研究。
(1) 为减少在整个浇注阶段由于钢液吸气造成的二次氧化,除了对中间包结构进行优化设计外,同时应当研究不同成分钢液的吸氧速率。对于浇注含有易与SiO2发生剧烈化学反应的合金元素(如Al、Ti、Mn和稀土元素)的钢种,可通过设计新型覆盖剂成分降低SiO2活度以提高熔渣对夹杂物的吸收作用并减轻对钢液的氧化作用,在设计覆盖剂成分时也应当考虑覆盖剂对中间包耐火材料的侵蚀。在中间包和水口耐火材料成分及结构设计时,应减少中间包耐火材料中不稳定氧化物含量和水口材料释放的CO气体,可以减轻钢液的二次氧化和延缓水口结瘤。
(2) 整个浇注过程涉及钢液的多相流动和温度的变化,建立钢液-空气-渣-夹杂物-耐火材料多相流动和传热耦合数学模型是模拟中间包内钢液二次氧化过程的关键,可以对整个浇注过程中元素和夹杂物成分的变化做到全面的分析。同时这一过程受到热力学和动力学等参数的共同影响,且非稳定和稳定浇注过程中钢液发生二次氧化的机理也不相同。目前的研究成果对中间包内二次氧化机理研究还不够深入,数学模型还不够完善,对中间包内钢液成分和夹杂物成分变化的准确预报还需要进一步研究。
参考文献
Low-carbon development of steel industry
[J].
钢铁工业低碳化发展
[J].从钢铁制造流程动态运行的物理本质出发,论述了钢铁工业低碳化发展的物理本质——制造流程、供应链和服务链运行过程中耗散结构的合理构建和耗散过程的优化,认为钢铁工业低碳化发展是一个系统性命题,不仅要从具体的工序/装置来解决,更重要的是要从流程结构、流程功能、流程效率等方面来解决。进而,基于国内外钢铁行业CO<sub>2</sub>排放现状的分析,构建了中国钢铁行业碳达峰与碳中和的情景分析模型,通过情景分析,量化分析了主要降碳措施的减排贡献,指出在钢铁行业实现碳达峰与碳中和的过程中,粗钢产量控制的累计减排贡献约为45%,有序、合理地利用废钢约占39%,氢还原技术约占9%,节能、“界面”技术、智能化等因素约占7%。同时,提出了中国钢铁行业低碳发展路线图的设想和碳达峰平台期、脱碳化、碳中和3个阶段的发展目标,并从资源、能源、生产制造流程脱碳化3个角度对未来钢铁行业的发展前景进行了展望,分析表明,到2060年,中国钢铁行业的铁矿石消耗量将有望减少75%,废钢的利用量将增加89%;煤炭消耗量将有望减少92%,电力消耗量的波动相对较小,氢气的用量将可能达到1 400万t/a;高炉-转炉长流程占比约为15%,全废钢电炉流程约为60%,氢还原-电炉流程约为25%。最后,提出了未来钢铁行业3类流程的设想,即高炉-转炉长流程、全废钢电炉流程和氢还原-电炉流程。
Evolution and control of non-metallic inclusions in steel during secondary refining process
[J].The problem of inclusions is one of the key concerns in the production process of high-quality special steel grades. This study summarized the main inclusion types, and their formation, evolution, and removal mechanisms during the secondary refining process. Meanwhile, combined with studies and practices of the authors, some control measures of inclusions were also discussed. According to this study, the inclusion types after refining are generally different from that of initial deoxidation products, and the formation and evolution of these inclusions are closely related to the dissolved elements in liquid steel, e.g., Ca, Mg, and Ti. Although sometimes the compositions of the inclusions are the same, their different shapes and distributions can also lead to different grades of inclusions depending on the micrographic method. Overall, solid inclusions can be easily removed compared with liquid inclusions, and Al2O3 and MgO·Al2O3 inclusions have a higher removal efficiency in contrast to liquid CaO-Al2O3 system inclusions. Refining slag, refractory, and ladle glaze may have a great impact on the control of trace elements and evolution of inclusions in liquid steel; therefore, suitable slag basicity and slagging operations are important during the refining process. In the case of Al-killed steel grades, slag with a basicity of 4-7 leads to a good deoxidation result, while the slag basicity adjustment during the refining process is generally negative for the control of inclusions in Si-Mn-killed steel grades. Moreover, special attention should be given to the use of CaO-containing refractory. High-quality clean alloys and a suitable alloying stage can also be beneficial for the control of trace elements and the removal of inclusions in the alloys. Furthermore, during the refining process, excessive stirring should be avoided to reduce the flush-off of ladle glaze, and inclusion modification technologies should be considered with precautions. Some methods, e.g., the control of Ca content, the prevention of slag entrainment, and the removal of ladle filler sands, are helpful for the control of micro-inclusions. Recent studies on the inclusions appropriately explained many phenomena in metallurgical processes, indicating some new directions for inclusion control. In the near future, certain mechanisms (e.g., the growth of CaO-Al2O3 inclusions) still need further investigation, and some new technologies are also required to solve the known problems, e.g., complete removal of ladle filler sands.
钢精炼过程非金属夹杂物演变与控制
[J].夹杂物控制是高品质特殊钢生产的难点和重点,本文介绍了钢中夹杂物的主要类型及其在精炼过程中的生成演变及去除行为规律,并结合作者的研究与实践,阐述了夹杂物控制的关键技术。钢中夹杂物类型与最初脱氧产物有较大的区别,其生成和演变与钢中的成分元素(如Ca、Mg和Ti等)密切相关,同一组成的夹杂物在钢中形态分布不同,最终会导致评级类别的差异。精炼过程中固态夹杂物通常比液态夹杂物更容易被去除,Al<sub>2</sub>O<sub>3</sub>和MgO·Al<sub>2</sub>O<sub>3</sub>夹杂物相比液态的CaO-Al<sub>2</sub>O<sub>3</sub>系夹杂物具有更高的去除效率。精炼渣、耐火材料以及钢包挂渣均会对钢中微量元素控制和夹杂物演变行为产生重要影响,适宜的渣碱度和稳定的造渣操作对夹杂物的控制非常重要。铝镇静钢的精炼渣碱度控制在4~7之间即可获得较好的脱氧效果;而硅锰镇静钢精炼渣采用变碱度操作并不利于夹杂物控制。同时,应该谨慎使用含CaO耐火材料。优质的洁净合金以及适宜的合金化时机,有助于控制钢中微量元素,减少合金中夹杂物的污染。精炼过程应避免过度搅拌,减弱对钢包挂渣的冲刷,并理性考虑夹杂物变性处理。控制钢中Ca含量、避免卷渣以及尽可能排除引流砂等手段有助于控制钢中的大型夹杂物。近年来的夹杂物演变和去除机理研究解释了诸多冶金过程现象,并提出了夹杂物控制新方向,但仍有如CaO-Al<sub>2</sub>O<sub>3</sub>夹杂物长大机制等机理需要进一步研究揭示,也亟待开发新的夹杂物控制技术来解决诸如如何彻底消除引流砂大型夹杂物等已知问题。
Formation and prevention of nozzle clogging during the continuous casting of steels: A review
[J].
State of the art in evaluation and control of steel cleanliness
[J].
Reoxidation phenomena of liquid steel in secondary refining and continuous casting processes: A review
[J].
Effect of rice husk ash insulation powder on the reoxidation behavior of molten steel in continuous casting tundish
[J].
Reoxidation behavior of molten steel in tundish
[J].
Tundish open eye formation: A trivial event with dire consequences
[J].
Quantitative analysis of contamination of molten steel in tundish
[J].
Technology for cleaning of molten steel in tundish
[J].
Effect of stirring on oxidation rate of molten steel
[J].
Oxidation rate of molten steel by argon gas blowing in tundish oxidizing atmosphere
[J].
Influence of steel grade on oxidation rate of molten steel in tundish
[J].
Numerical simulation and application of tundish cover argon blowing for a two-strand slab continuous casting machine
[J].
Research and application progress of plasma heating technology for continuous casting tundish
[J].In the steel industry is facing the new normal situation of transformation and upgrading, with the help of intelligent equipment to drive the steel industry to green and high-end development is expected to achieve the optimization of the steel production organization. Continuous casting tundish with constant temperature and low superheat can effectively improve the quality of steel, so it is necessary to develop the heating technology of tundish. Therefore, tundish heating temperature control technology has been paid more and more attention. Regarding to the hot issues of tundish plasma heating technology, which has attracted increasing attention in recent years, systematically describes the heating principle and equipment characteristics, introduces the research and development of equipment and metallurgical functions of plasma heating technology at home and abroad, and mainly analyzes the influence of plasma heating technology on the flow field, temperature field, inclusion removal and chemical composition of steel liquefication in tundish, and the metallurgical effect of practical application. Based on the deep understanding of the research and application of plasma heating technology, the new problems found in the heating process of a new type of hollow graphite electrode plasma equipment independently developed in China are discussed, and the ways to further improve its metallurgical effect are discussed. Analysis indicates that the domestically developed hollow graphite electrode plasma heating equipment is better suited to meet the transformation needs of China's steel industry. This equipment provides an effective solution to issues such as the instability of superheat in casting steel, lower cleanliness levels in molten steel, and uneven composition of molten steel. It addresses the shortcomings in the intelligent positioning of the "one-key heating" intermediate package, thus enhancing its precision.
中间包等离子体加热技术研究进展及应用
[J].
Developing of the technology of tundish metallurgy
[J].
中间包冶金技术的发展
[J].
Active tundish slag
[J].
Nozzle clogging behaviour of Ti-bearing Al-killed ultra low carbon steel
[J].
Investigation on the effects of ladle change operation and tundish cover powder on steel cleanliness in a continuous casting tundish
[J].
In situ observation of the dissolution of SiO2 particles in CaO-Al2O3-SiO2 slags and mathematical analysis of its dissolution pattern
[J].
Concept of inclusion capacity of slag and its application
[J].
精炼渣的夹杂物容量的概念及其应用
[J].分析了目前文献中使用夹杂物组分在渣中的饱和质量分数和当前质量分数的差值除以渣的黏度(ΔC/η)这一参数来评价夹杂物在渣中溶解能力的文献来源以及该参数的局限性,包括该参数有量纲且没有包含夹杂物尺寸的影响。与流体流动的自然对流传热中使用的格拉晓夫数进行类比,提出了渣的夹杂物容量的概念,即无量纲的Zh数,同时提出了无量纲溶解速度R<sub>y</sub>的概念,得到了渣的夹杂物容量与夹杂物无量纲溶解速度之间的关系。夹杂物容量可以用来预测夹杂物在渣中的溶解速度和溶解时间,可以用来计算溶解过程化学反应系数和夹杂物组分在渣中的扩散系数,还可以用来计算夹杂物从钢液中的去除时间。随着夹杂物容量Zh数的增加和夹杂物尺寸的增大,夹杂物的溶解时间增大。随着夹杂物尺寸的增大,夹杂物的平均去除时间增加而最大去除时间降低。温度为1 600 ℃时,针对Al<sub>2</sub>O<sub>3</sub>夹杂物在CaO-Al<sub>2</sub>O<sub>3</sub>-SiO<sub>2</sub>渣中的溶解过程,计算得到的界面化学反应系数为5×10<sup>-6</sup>~10×10<sup>-6</sup> m/s,扩散系数为2.5×10<sup>-10</sup>~4×10<sup>-10</sup> m<sup>2</sup>/s,其值依据渣成分的变化而变化。
Steel reoxidation by gunning mass and tundish slag
[J].
Prediction of re-oxidation behaviour of ultra-low carbon steel by different slag series
[J].A kinetic model was developed using FactSage Macro Processing to simulate the re-oxidation of ultra-low carbon steel via different oxidising slags. The calculated results show good agreement with experimental laboratory thermal simulation data. Therefore, the model can be used to predict the change behaviour of slag-metal-inclusion in the re-oxidation reaction of liquid steel. It can provide prediction and guidance for an accurate secondary oxidation control process. During the slag re-oxidation process, when the oxygen in the steel is supersaturated and the slag is low in oxidation, it can easily form stick-like and dendritic shape inclusions of AlO in steel. As the (FeO) content increases in slag, the oxygen transfer from slag to steel is evident, and the inclusion size increases, showing clusters and spherical shapes. In addition, supersaturated oxygen in steel easily forms unstable AlO-TiO inclusions with [Ti]. As the components of liquid steel tend to be uniform, the AlO-TiO inclusions will decompose and disappear, forming stable AlO and TiO inclusions. The number of inclusions can be reduced by increasing the basicity and the ratio of CaO to AlO in the initial slag.
Reoxidation behavior of molten steel in non-killed and Al-killed steels
[J].
Removal of inclusion from molten steel in continuous casting tundish
[J].
Influence of calcium aluminate flux on reoxidation behaviour of molten steel during continuous casting process
[J].
Reoxidation of Al-killed steel by Cr2O3 from tundish cover flux
[J].
A kinetic model of mass transfer and chemical reactions at a steel/slag interface under effect of interfacial tensions
[J].
Reoxidation behavior in Al killed steel during casting
[J].
Alキルド鋼鋳込時の溶鋼再酸化挙動
[J].
Laboratorial analysis of inclusions formed by reoxidation in tundish steelmaking
[J].
Effect of tundish flux on compositional changes in non-metallic inclusions in stainless steel melts
[J].
Effect of tundish flux on reoxidation behavior of Si-killed 316L stainless steel
[J].
Material evaluation to prevent nozzle clogging during continuous casting of Al killed steels
[J].
Mechanism of alumina deposition on alumina graphite immersion nozzle in continuous caster
[J].
Control of MgO·Al2O3 spinel inclusions in stainless steels
[J].
Mechanisms of inclusion evolution and SEN clogging in ultra-pure ferritic stainless steels
[D].
超纯铁素体不锈钢夹杂物演变与浸入式水口结瘤机理研究
[D].
Reaction mechanism between alumina graphite immersion nozzle and low carbon steel
[J].
Mechanism of deposition of inclusion and metal in ZrO2-CaO-C immersion nozzle of continuous casting
[J].
In-situ measurement of gas emission by pyrolysis of various ceramic materials used for submerged-entry nozzle refractory
[J].
Effects of carbon and silica in submerged entry nozzles on alumina buildup
[J].
松井 泰次郎, 池本 正, 澤野 清志
Oxidation of Ti added ULC steel by CO gas simulating interfacial reaction between the steel and SEN during continuous casting
[J].
Growth of initial clog deposits during continuous casting of Ti-ULC steel—Formation and reduction of the initial deposits at nozzle/steel interface
[J].
Quantitative assessment of microporous MgO castable erosion and corrosion behaviors in two tundish covering fluxes
[J].
Simultaneous enhance of the thermal shock resistance and slag-penetration resistance for tundish flow-control refractories: The role of microporous magnesia
[J].
Preparation and properties of sol-bonded magnesia-calcia hot gunning mixes
[D].
溶胶结合镁钙质热态喷补料的制备及性能研究
[D].
Interaction between steel and distinct gunning materials in the tundish
[J].
Fundamentals of interfacial wettability in ironmaking and steelmaking
[J].
钢铁冶金过程中的界面润湿性的基础
[J].
Effect of the tundish gunning materials on the steel cleanliness
[J].
Reoxidation of Ni- and Ni-Fe-alloys by Al2O3-SiO2 refractory materials
[J].
Interaction between molten steel and different kinds of MgO based tundish linings
[J].
Mechanism of interface reactions between Fe-2%Al alloy and high-silica tundish refractory
[J].
Effect of interactions between Fe-Al alloy and MgO-based refractory on the generation of MgO·Al2O3 spinel
[J].
Reaction behavior of high manganese and high aluminum steel with chromium-containing ladle filler sand
[J].
Laboratory investigation on quantitative effect of ladle filler sands on the cleanliness of a bearing steel
[J].
Analysis on source of MnO/FeO containing macro-inclusions in alloyed steel
[J].
合金钢中MnO/FeO大型夹杂物来源分析
[J].为了研究钢中MnO/FeO大型夹杂物的来源,基于工业试验和大样电解分析,对比了钢中的显微夹杂物和引流砂的烧结行为。研究结果表明,钢中MnO/FeO大型夹杂物与细小显微夹杂物没有明显关联,这些大型夹杂物并不是来源于钢中的细小夹杂物。依据热力学分析可知,这些夹杂物也不是二次氧化的产物。基于铬质引流砂烧结机理和大型夹杂物的化学成分,可以确定这些大型夹杂物为引流砂及其烧结产物。当引流砂进入钢液后,要在中间包内完全去除是十分困难的。在钢包开浇时,应尽可能移除引流砂,这对提升钢的质量具有重要意义。
Improvement in cleanness of continuously cast slab by decreasing slag carry over
[J].
减少钢包下渣提高铸坯洁净度
[J].
Influence of reoxidation in tundish on inclusion for Ca-treated Al-killed steel
[J].
Inclusion control with Ca treatment to improve castability of low carbon aluminum-killed steel
[A].
Significance of nonmetallic inclusions for the clogging phenomenon in continuous casting of steel——A review
[J].
Effect of reoxidation on inclusions in steel during calcium treatment
[J].
Control of inclusion composition in calcium treated aluminum killed steels
[J].
A reaction model for prediction of inclusion evolution during reoxidation of Ca-treated Al-killed steels in tundish
[J].
A thermodynamic model to predict the composition of inclusions in Al-killed Ca-treated steels
[J].
A review of steel processing considerations for oxide cleanliness
[J].
Effect of reoxidation on inclusions characteristic during casting in Al-killed steel containing rare earth
[J].
Influence of reoxidation and calcium treatment on nonmetallic inclusions in ultra-low oxygen special steel
[J].
二次氧化及钙处理对超低氧特殊钢中非金属夹杂物的影响
[J].
Evolution of nonmetallic inclusions in GCr15 bearing steels during continuous casting process
[J].
Formation and evolution of inclusions in AH36 steel during LF-RH-CC process: The influences of Ca-treatment, reoxidation, and solidification
[J].
Evolution of non-metallic inclusions in a 303-ton calcium-treated heavy ingot
[J].
Effect of reoxidation on inclusions in Al-killed stainless steel during the casting start process
[J].
开浇过程二次氧化对铝脱氧不锈钢中夹杂物的影响
[J].
Evolution of inclusions at different degrees of secondary oxidation in GCr15 bearing steel
[J].In order to study the effect of secondary oxidation on inclusions in bearing steel, an iron sampler was used to sample the bearing steel liquid after RH breaking. At the same time, different qualities of wellpacking sand or FeO powder were added to different iron samplers before sampling to simulate different degrees of oxidation of the steel liquid. The research results indicate that the inclusions in the steel during RH breaking are mainly calcium aluminate. When the steel undergoes slight oxidation, the total oxygen mass fraction increases to (0.3-1.0)×10-6, MgO·Al2O3 inclusions can be detected in steel; CaO·6Al2O3 and Al2O3 inclusions are observed with further oxidation. When the total oxygen mass fraction of molten steel increases by more than 6 ×10-6, the MgO content in the inclusions will significantly decrease with the formation of a large amount of Al2O3 inclusions, resulting in the detection of MgO·Al2O3 inclusions in the steel. The industrial results show that the total oxygen mass fraction of molten steel in the casting process increases by (0.3—1.5)×10-6 compared to that of the end of RH vacuum, and many MgO·Al2O3 inclusions can be detected in the tundish. The number of MgO·Al2O3 inclusions in the casting zone of the tundish is significantly higher than that in its impact zone, indicating that the oxidation that occurs during the flow of molten steel in the casting area is the main link in the oxidation of molten steel in the tundish..
GCr15轴承钢不同二次氧化程度下的夹杂物演变规律
[J].为了研究二次氧化对轴承钢夹杂物影响,采用铁质取样器对RH破空后轴承钢钢水进行取样,同时取样前在不同铁质取样器中加入不同质量的铬质引流砂或FeO粉,以模拟钢水不同程度的氧化。研究结果表明,RH破空时钢中夹杂物以钙铝酸盐为主,当钢水发生轻微的氧化,总氧质量分数增加(0.3~1.0)×10-6,可以在钢中检测到许多MgO·Al2O3夹杂物;随着氧化程度的提高,还伴有CaO·6Al2O3和Al2O3夹杂物生成;当钢水总氧质量分数增加值超过6×10-6,夹杂物中MgO含量会随Al2O3夹杂物大量生成而显著降低,导致钢中检测不到MgO·Al2O3夹杂物。工业结果表明,浇铸过程中间包钢水总氧质量分数相比RH真空结束增加(0.3~1.5)×10-6,此时可以在中间包钢水中检测到许多MgO·Al2O3夹杂物,并且中间包浇铸区MgO·Al2O3夹杂物数量显著多于冲击区,说明浇铸区内钢水流动过程中发生的氧化是中间包钢水氧化的主要环节。
A study on the transient inclusion evolution during reoxidation of a Fe-Al-Ti-O melt
[J].
Behavior of non-metallic inclusions of IF steel during production process
[J].
IF钢生产过程非金属夹杂物行为研究
[J].对首钢京唐生产IF钢的同一浇次前2炉的RH精炼、镇静和中间包浇铸过程进行了系统取样,并利用Aspex自动扫描电子显微镜分析统计了钢中夹杂物的成分、尺寸等信息.研究发现,Al<sub>2</sub>O<sub>3</sub>-Ti<sub>○</sub>xO复合夹杂物在Ti合金化和二次氧化的情况下都会生成,并随着精炼的进行逐渐转变为Al<sub>2</sub>O<sub>3</sub>,这与热力学计算的结果一致;而Al<sub>2</sub>O<sub>3</sub>可以作为Al<sub>2</sub>O<sub>3</sub>-Ti<sub>○</sub>xO的形核核心,形成Al<sub>2</sub>O<sub>3</sub>-Ti<sub>○</sub>xO包裹Al<sub>2</sub>O<sub>3</sub>的夹杂物,并且在Al<sub>2</sub>O<sub>3</sub>-Ti<sub>○</sub>xO转变为Al<sub>2</sub>O<sub>3</sub>的过程中会导致钢滴进入夹杂物内部,从而形成Al<sub>2</sub>O<sub>3</sub>包裹钢滴的夹杂物.
Evolution of non-metallic inclusions in Al-killed stainless steelmaking
[J].
Transient behavior of inclusions during reoxidation of Si-killed stainless steels in continuous casting tundish
[J].
Understanding the formation and evolution of oxide inclusions in Si-deoxidized spring steel
[J].
Evolution of non-metallic inclusions in Si-killed stainless steelmaking
[J].
Reassessment of oxide stability diagram in the Fe-Al-Ti-O system
[J].
Influence of Al/Ti ratio in Ti-ULC steel and refractory components of submerged entry nozzle on formation of clogging deposits
[J].
Modeling of liquid steel/slag/argon gas multiphase flow during tundish open eye formation in a two-strand tundish
[J].
Numerical simulation on the multiphase flow and reoxidation of the molten steel in a two-strand tundish during ladle change
[J].
Simulation of gas-liquid two-phase flow in metallurgical process
[J].The metallurgical process involves complex phenomena comprising high temperature, the multiphase flow, and the physical and chemical reactions in the process reactors. Because of the complexity of the metallurgical process and the limitation conditions for the direct measuring and observation, numerical and physical simulations have become indispensable and effective tools to analyze and reproduce the transport phenomena and mechanisms occurring in the process. Transport phenomena of the gas-liquid two-phase flow plays a dominant role in process metallurgy since their respective movement laws govern the kinetics of the various physical phenomena in the metallurgical reactors. The gas-liquid two-phase flow has complex interface structures, and the accuracy of the interfacial momentum transfer models, including the interfacial forces, which is one of the keys to predicting the distribution of gas phase in the two-phase flow system successfully. This paper is aiming at reviewing the two-phase flow models based on the Euler-Euler system, the interfacial force model, and the turbulence model for gas-liquid two-phase flow. The use and extent of numerical and physical simulation for transport phenomena of two-phase flow in the steelmaking and casting processes are summarized and explored, including the basic oxygen furnace, electric arc furnace, refining, tundish, and molds. The methods and typical application in the numerical and physical simulation of gas-liquid two-phase flow will provide useful guides for the research.
冶金过程中的气液两相流模拟
[J].
Numerical simulation on optimization of molten steel flow field of continuous casting tundish
[D].
连铸中间包钢液流场优化的数值模拟研究
[D].
Novel application of alkali oxides in basic tundish fluxes for enhancing inclusion removal in 321 stainless steels
[J].
Improving cleanliness of 16MnCrS5 case hardening steels by optimized active tundish flux
[J].
Comparison study on effect of nano-sized Al2O3 addition on the corrosion resistance of microporous magnesia aggregates against tundish slag
[J].
In situ observation of the direct and indirect dissolution of MgO particles in CaO-Al2O3-SiO2-based slags
[J].
Kinetics of spinel formation and growth during dissolution of MgO in CaO-Al2O3-SiO2 slag
[J].
In-situ observation of slag penetration into MgO refractory
[J].
Thermodynamic corrosion behavior of Al2O3, ZrO2 and MgO refractories in contact with high basicity refining slag
[J].
Corrosion modeling of magnesia aggregates in contact with CaO-MgO-SiO2 slags
[J].
MgO solubility in steelmaking slags
[J].
Wetting and corrosion behavior of MgO substrates by CaO-Al2O3-SiO2-(MgO) molten slags
[J].
Corrosion behavior of lightweight MgO in high basicity tundish slag
[J].
Formation mechanism of interface reaction layer between microporous magnesia refractories and molten steel and its effect on steel cleanliness
[J].The ceramic filter in continuous casting tundish can effectively improve the cleanliness of high-performance steel by regulating tundish flow field to promote the removal of inclusions and adsorbing or blocking fine inclusions in the molten steel into the mold. The interaction between microporous magnesia refractories used as tundish filter and molten interstitial- free (IF) steel at 1873 K was investigated to reveal the formation mechanism of their interface layer and its effect on steel cleanliness by laboratory research and thermodynamic calculations. The results show that the magnesium–aluminum spinel layer at the interface between the molten IF steel and the microporous magnesia refractories is formed mainly by the reaction of MgO in the refractory with the [Al] and [O] in the molten steel, significantly reducing the total O content, the size and amount of inclusions of the molten steel. In addition, the interparticle phases of microporous magnesia refractories at high temperature can adsorb Al2O3 and TiO2 inclusions in the molten steel into interparticle channels of the refractories to form high melting point spinel, impeding the further penetration of the molten steel. As a result, the consecutive interface layer of high melting point spinel between microporous magnesia refractories and molten steel can improve the cleanliness of the molten steel by adsorbing inclusions in the molten steel and avoid the direct dissolution of refractories of the tundish ceramic filter immersed in the molten steel, increasing their service life.
Design, fabrication and properties of lightweight wear lining refractories: A review
[J].