可充电镁电池负极材料及界面化学的研究进展
Research Progress on Anode Materials and Interfacial Chemistry for Rechargeable Magnesium Batteries
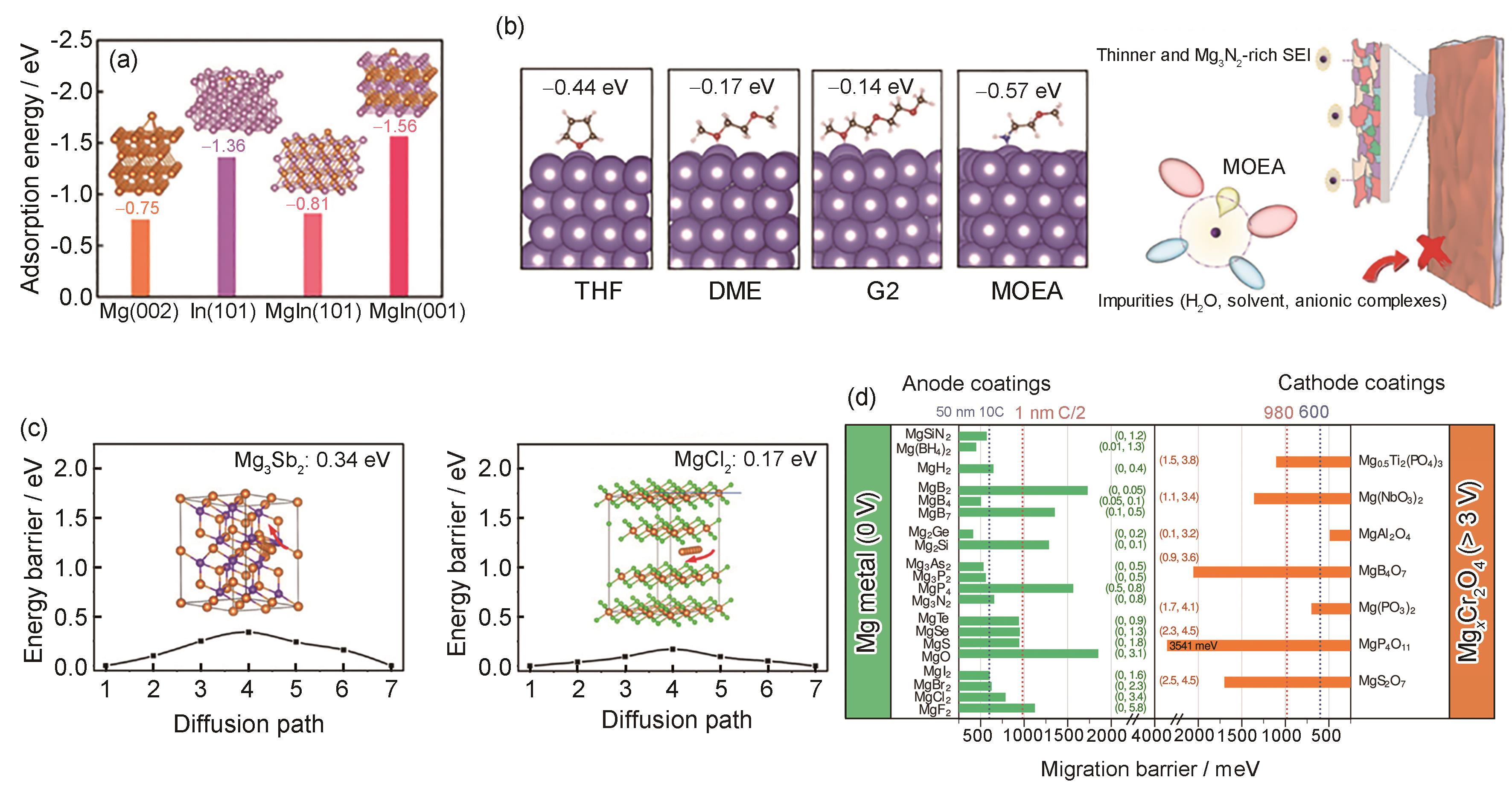
(a) calculated adsorption energies of Mg atoms onto different Mg and MgIn crystal facets[
(b) adsorption models and energies of THF, glyme (DME), G2, and 2-methoxyethylamine (MOEA) molecules on Mg (0001)[
(c) calculated Mg migration energy barriers in bulk Mg3Sb2 and MgCl2[
(d) summary of the calculated diffusion barriers of Mg2+ in various inorganic Mg compounds[