非平衡界面动力学理论
|
|
王海丰, 蒲振新, 张建宝
|
Non-Equilibrium Interface Dynamics Theory
|
|
WANG Haifeng, PU Zhenxin, ZHANG Jianbao
|
|
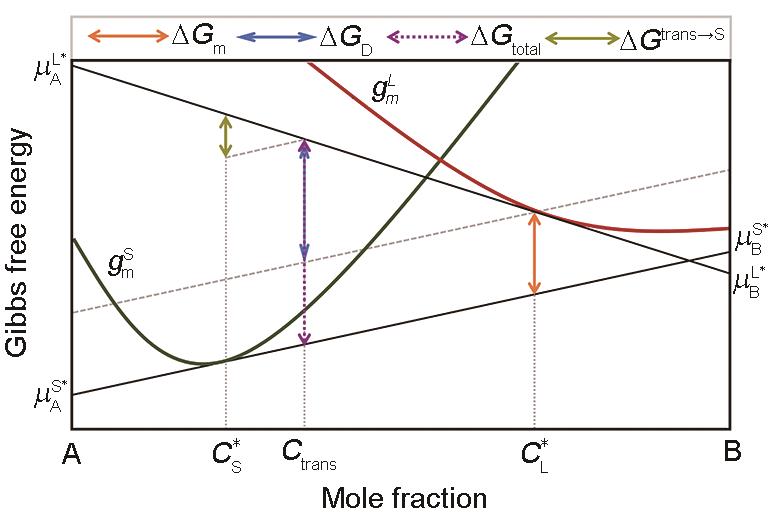
图4 非稳态固/液界面动力学过程的摩尔Gibbs自由能示意
|
Fig.4 Mole Gibbs free energy diagram for the solid/liquid interface kinetic processes under a non-steady-state condition (Total Gibbs free energy dissipated by the interface after solidification of 1 mol liquid is = . By translating the tangent of Gibbs free energy curve of solid at to the Gibbs free energy curve of liquid at , Gtotal is divided into two parts: The upper one for trans-interface diffusion is = and latter part for interface migration is = . The difference in Gtotal between the steady-state condition and the non-steady-state condition is = , which is the Gibbs free energy dissipated to adjust the actual composition transferred across the interface from to Ctrans (—the solute component that finally crosses the interface and enters the solid phase)
|
|
|
|
|

