镁基储氢合金动力学调控及电化学性能
|
|
朱敏, 欧阳柳章
|
Kinetics Tuning and Electrochemical Performance of Mg-Based Hydrogen Storage Alloys
|
|
ZHU Min, OUYANG Liuzhang
|
|
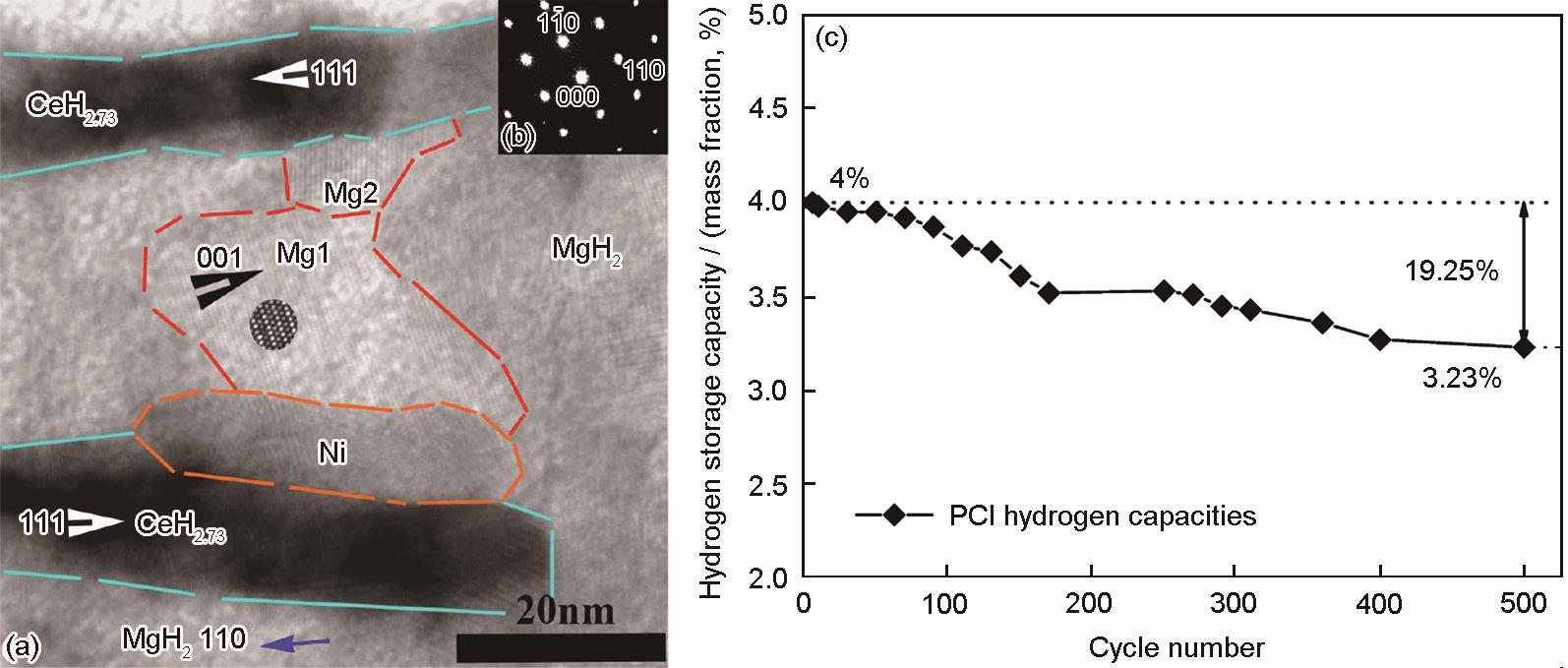
图2 部分放氢的MgH2-Mg2NiH4-CeH2.73纳米复合储氢材料中,MgH2首先在CeH2.73、Ni和MgH2界面处发生分解并形成Mg的TEM明场相、MgH2选区电子衍射花样,及CeH2.73-MgH2-Ni复合材料最大储氢容量与循环次数关系图[43]
(a) bright field image of the in situ formed CeH2.73-MgH2-Ni composite
(b) selected area electron diffraction pattern of MgH2 (zone axis []). Mg nuclei preferentially nucleate along the surface of CeH2.73/CeH2 and Ni phase at the starting transition stage of MgH2 to Mg during the dehydrogenation process
(c) evolution of the maximum hydrogen sorption capacities versus cycle times of CeH2.73-MgH2-Ni composite
|
Fig.2 TEM image and analyses of the microstructure of the partially dehydrogenated CeH2.73-MgH2-Ni nanocomposites demonstrating the catalyst effect of CeH2.73 and Ni on MgH2 dehydrogenation process[43]
|
|
|
|
|
